INTRODUCTION
Heart failure (HF) is a clinical syndrome that results from either the impairment of ventricular filling or the ejection of blood that then causes dyspnea and fatigue with decreased exercise tolerance, fluid retention, pulmonary congestion, splanchnic congestion, and peripheral edema. HF is estimated to affect 5.8 million Americans and is primarily a disease of the elderly. It has an increasing prevalence with age [1], reaching 17.4% in those 85 or older.
HF is expensive and deadly. In the USA, there was an estimated 30.7 billion dollars spent in 2012 on the care of HF with projections to more than double by 2030 [2]. Further, approximately 50% of patients will die within 5 years of diagnosis [3] and 30-day, 1-year, and 5-year mortality after hospitalization for HF are reported at 10.4%, 22%, and 42.3%, respectively [4]. HF is also the most common cause of acute dyspnea among elderly patients in the emergency department (ED) [5] and the most common cause of death among patients presenting to the ED with dyspnea [6].
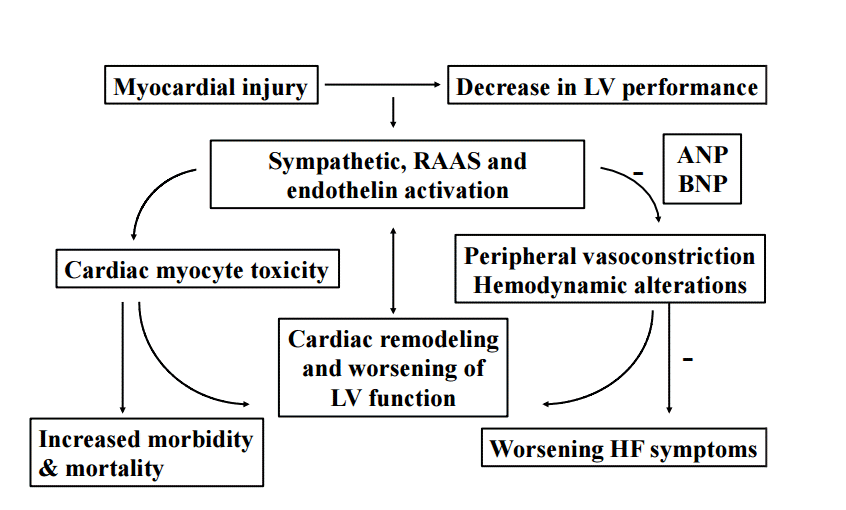
HF is the common result of impaired left ventricular function (Fig. 1) and is associated with a large number of comorbidities. These include hypertension, diabetes, coronary artery disease, valvular and other cardiovascular structural diseases. Although most patients have symptoms due to impaired left ventricular function, HF is not synonymous with cardiomyopathy or left ventricular dysfunction. Ejection fraction (EF) is considered important in the classification of HF for prognosis and response to therapies. It is important to note that HF is not synonymous with impaired systolic function, as patients with preserved EF may still manifest symptomatic HF. Thus, when stratifying HF patients by EF, it is preferable to use the terms HF with preserved EF and HF with reduced EF. Preserved EF refers to EF greater than or equal to 50%, reduced EF refers to EF less than or equal to 40% with EF 41% to 49% considered as patients with borderline preserved EF [7].
PRESENTATION
Patients typically present with dyspnea and/or increased peripheral edema. Patients may also complain of cough and fatigue and may notice a worsening of their baseline HF symptomology. This may manifest as increased dyspnea on exertion with decreased exercise tolerance, requiring more pillows to rest comfortably at night, or they may experience paroxysmal nocturnal dyspnea.
Not uncommonly patients may develop “flash” pulmonary edema. This occurs when acutely elevated left ventricular end diastolic pressure (LVEDP) and cardiac filling pressures result in rapid accumulation of fluid within the lung’s alveolar and interstitial spaces. Flash pulmonary edema has been associated with diastolic dysfunction, hypertensive crisis, renal artery stenosis, coronary artery disease, valvular heart disease, obstructive sleep apnea and Takotsubo cardiomyopathy, although any process that causes an acute increase in LVEDP may precipitate flash pulmonary edema [8,9].
EVALUATION
Early diagnosis is critical as delays in diagnosis lead to delays in treatment which has been associated with increased acute mortality [10]. Initial evaluation of the patient should always include a thorough history and physical examination which attempts to identify the potential etiology and precipitating factors for decompensation. Specific emphasis is placed on potential cardiac ischemia, fluid overload, medication and dietary compliance, as well as a detailed past medical history with attention to a history of severe hypertension, valvular heart disease, myocardial ischemia, atrial fibrillation and other arrhythmias, Takotsubo’s cardiomyopathy, drug and medication use, renal failure and renal artery stenosis.
A detailed history and physical is part of every patients presentation to the ED but may be expedited in unstable patients, and those with severe respiratory distress. History and physical are important steps in confirming the diagnosis as well as excluding other causes of dyspnea. However, history and physical have its limitations.
Classic symptoms for HF include dyspnea, dyspnea on exertion, and orthopnea. However, dyspnea and orthopnea have overall poor sensitivity and specificity for the diagnosis of HF. Dyspnea, with a 56% sensitivity and 53% specificity for a HF diagnosis is diagnostically worthless. Orthopnea only performs marginally better in specificity but loses in sensitivity, being 77 and 50% respectively. Ultimately, a history of prior HF performs singly better than either of these typical presenting symptoms, with a sensitivity of 60% and a specificity of 90% [11].
Typical findings associated with acute decompensated heart failure (ADHF) include jugular venous distention or distended neck veins, peripheral edema, pulmonary crackles, and an S3 on heart examination. Edema has a sensitivity and specificity of 50% and 78%. Crackles on lung exam have a sensitivity of 60% and specificity of 78% while jugular venous distention has a sensitivity and specificity of 39% and 92% [11]. The S3 remains one of the best clinical indicators for HF. Although the sensitivity is low at 13%, its specificity of 99% should confirm a suspected diagnosis of HF in the dyspneic patient, albeit it may be difficult to auscultate in a busy and noisy ED. It is notable that the majority of HF signs and symptoms have low sensitivity overall but perform better with regards to specificity and thus are better tests for ruling in a HF diagnosis as opposed to ruling it out. Table 1 summarizes the sensitivity and specificity for HF of these and other findings in patients presenting to the ED with dyspnea [11].
An electrocardiogram (ECG) should be performed shortly after presentation on all dyspneic patients and those with a suspicion of HF. Although it does not typically contribute directly to the diagnosis of HF, ECG testing can identify several precipitants and may create multiple branch points in patient management. Patients with dyspnea and underlying cardiac ischemia or arrhythmia can be quickly identified and receive early treatment based on the results of ECG testing. ECG findings prompting immediate treatment may include ST segment elevation myocardial infarction, atrial fibrillation with rapid ventricular response, or primary ventricular arrhythmias.
The evaluation of the dyspneic patient and the patient with potential HF also typically includes a chest X-ray. Although commonly non-diagnostic for HF, this can frequently identify other causes of dyspnea such as pneumonia, or findings consistent with chronic obstructive pulmonary disease, or may provide evidence to support the diagnosis of HF such as cardiomegaly or overt signs of pulmonary edema with central vascular congestion. However, the chest X-ray is similar to the history and physical where a normal chest X-ray does not exclude HF as the presenting diagnosis [12].
Bedside ultrasound (US) is an emerging study in the evaluation of potential HF patients. Anderson et al. [13] looked at the utility of bedside US in the diagnosis of HF using 3 modalities. This study evaluated the combination of cardiac US for left ventricular EF, inferior vena cava collapsibility and pulmonary interstitial edema (i.e., B lines) to establish a HF diagnosis. The combination of all three modalities resulted in a poor sensitivity (36%), but an excellent specificity (100%; 95% confidence interval, 95 to 100). In addition any combination of 2 of the modalities had a specificity of 93% or greater. Thus, similar to some history and physical parameters, US has a high specificity and can confirm the presence of HF, but its low sensitivity precludes the ability to rule out its presence. Bedside US performed by EM physicians is rapidly expanding, although its use in the acutely dyspneic patient can be limited due to availability and discomfort associated with performing the above measures in a supine position.
LABORATORY TESTING
Due to the potential limitations and delays in the diagnosis of HF from historical measures clinicians have searched for a test that could rapidly diagnose or exclude HF. In 2002 Maisel et al. [14] showed that a B-type natriuretic peptide (BNP) greater than 100 pg/mL was a better predictor of HF than clinical judgement, with an overall accuracy of 81% compared to 74% for clinical judgement.
The use of BNP testing is an important ED diagnostic adjunct. While levels less than 100 pg/mL reliably exclude the diagnosis of HF, levels above 400 pg/mL are consistent with the diagnosis of HF. This ultimately leaves a grey zone, from 100 to 400 pg/mL that requires additional testing to determine an accurate diagnosis [15,16]. N-terminal pro BNP (NTproBNP), a metabolic byproduct of BNP synthesis, performs in a similar fashion diagnostically. Levels of NTproBNP below 300 pg/mL exclude a diagnosis of HF, and levels above 900 pg/mL are highly suggestive, with a 300 to 900 pg/mL gray zone requiring additional confirmatory testing [17,18]. NTproBNP also requires adjustment for age, with a rule in cutpoint of 1,800 pg/mL in patients older than 75 years. Importantly, natriuretic peptide (both BNP and NTproBNP) testing can be negatively confounded by the presence of obesity [19], resulting in a lowering of expected compared to the measured value. They are also positively confounded by the presence of renal failure [20]. Thus it has been recommended that in patients with a body mass index in excess of 35, the measured BNP levels should be doubled, and halved when in the presence of renal failure [21] (defined as a estimated glomerular filtration rate below 60 mL/min).
Other lab tests typically included in the evaluation are a basic metabolic panel with creatinine, and troponin. Hospitalized patients with an elevated troponin have a markedly higher acute mortality as compared to those with a troponin below the local cutpoint [22] and thus are candidates for more aggressive therapy. Furthermore, when a higher sensitivity troponin assay is available (Verisens RUO Human cTnI Assay; Nanosphere, Northbrook, IL, USA), those found to have a persistently elevated or rising troponin have much higher 90 day mortality and re-hospitalization rates and should have therapy to address these potential outcomes [23].
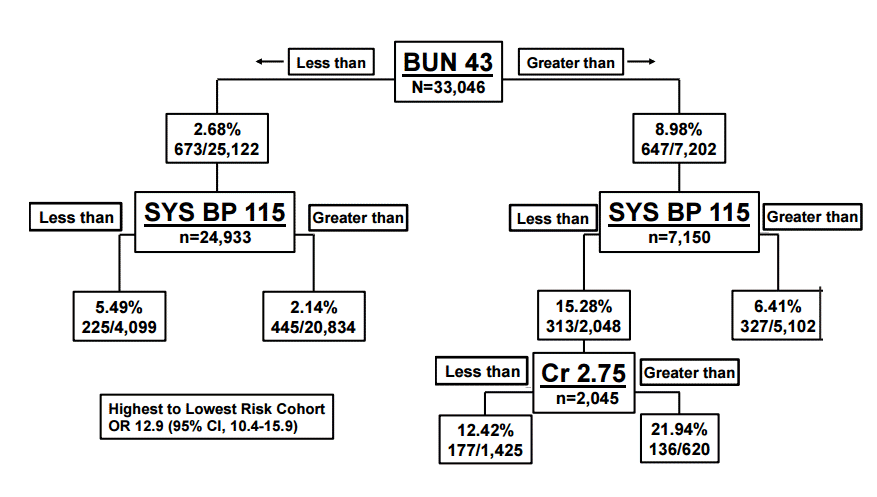
Renal function is important to measure in acute decompensated HF patients as renal function is a major predictor of mortality and severity of disease. Acute decompensated HF or worsening HF may contribute to worsening renal function as well; often referred to as cardiorenal syndrome. A decreased glomerular filtration rate is one of the strongest predictors of short term mortality, and it has been shown to represent an approximate 7% increase risk in mortality for every 10 mL/min decrease in glomerular filtration rate [24,25]. At least one in four patients admitted to the hospital for HF has a glomerular filtration rate less than 60 mL/min/1.73 m2) and patients who experience worsening renal function are associated with longer stays and both increased short and long term mortality [26] (Fig. 2).
The differential for acute dyspnea and acute decompensated HF is long and diverse. Efforts should focus on exclusion of other causes of dyspnea and their major treatment branch points, while continuing to treat acute HF. Acute severe exacerbations of asthma and chronic obstructive pulmonary disease often mimic ADHF, and may occur simultaneously. Other mimics include large pulmonary embolus and severe pneumonia as well as the multiple causes of noncardiogenic pulmonary edema such as the acute respiratory distress syndrome, toxins, high altitude and opiate overdose. Acute coronary syndrome and dyspnea as an angina equivalent is also an important consideration in the differential. Table 2 lists the potential differential diagnosis that may be encountered.
TREATMENT
Once the diagnosis of HF is established, treatment should be administered rapidly. Maintenance therapy for HF has evolved over time. From the beneficial discovery of beta-blockers to angiotensin converting enzyme inhibitors and now the combination of an angiotensin receptor blocker with a neprilysin inhibitor, baseline therapy continues to progress and improve long term outcomes for HF patients [27,28]. Chronic treatments to reduce long term mortality of HF are not always indicated in the early treatment of ADHF and are not the focus of this review. However, maintenance of guideline directed medical therapy is indicated if there is no contraindication to their administration [7].
INITIAL EVALUATION AND STABILIZATION IN PATIENTS WITH ACUTE SEVERE SYMPTOMS
Treatment in the ED starts with the ABC (airway, breathing, and circulation), similar to all acute processes. The initial evaluation of the patient with suspected ADHF seeks to identify those with severe respiratory distress and potential respiratory failure, as well as patients with severe hypertension that is contributing to their symptoms. Primary goals in the patient with acute severe symptoms include support of oxygenation, ventilation, and hemodynamic stabilization. The need to secure or establish an airway is always a concern for every emergency physician evaluating a patient who presents with acute dyspnea. This includes not just the need for immediate intubation but includes assessment of potential difficulties in intubation and securing the airway if necessary, as well as support of oxygenation and ventilation.
FLASH PULMONARY EDEMA
Severe respiratory failure in ADHF is usually a result of flash pulmonary edema. Flash pulmonary edema occurs with acute increases in left ventricular end diastolic filling pressure and initial treatment focuses at its decrease. While flash pulmonary edema has been clinically associated with renal artery stenosis, coronary artery disease, particular blood pressure profiles, obstructive sleep apnea, diastolic dysfunction, valvular heart disease, and Takotsubo cardiomyopathy [8], symptom treatment precludes an initial diagnostic search. Elevated LVEDP leads to an increase in interstitial edema and ultimately increased alveolar fluid leading to hypoxia and dyspnea. Although flash pulmonary edema is a type of cardiogenic pulmonary edema, it is also associated with increased permeability of the capillary endothelium believed to be secondary to changes in the neurohormonal milieu involving the renin angiotensin system, increased catecholamines, increased endothelin-1 and decreased nitric oxide synthesis [8,9].
TREATMENT FOR FLASH PULMONARY EDEMA
Patients with severe respiratory distress, hypoxia or respiratory acidosis may be temporized by the use of noninvasive ventilation (NIV) as long as they are able to cooperate with the implementation. Patients unable to cooperate should be considered immediate candidates for endotracheal intubation. A quick clinical delineation can be made on initial evaluation of the patient. Patients that are speaking in one word sentences may be placed on NIV. Patients whose respiratory function does not allow even one word sentences, that are confused or obtunded require intubation.
Two kinds of NIV are commonly used and consist of continuous positive airway pressure (CPAP) and bilevel positive airway pressure. The physiologic differences between the two types of NIV have little bearing on the treatment of flash pulmonary edema. Importantly, neither intervention can be considered as a standalone therapy. Ergo, while their use may preclude immediate intubation, their value is to buy time for the implementation of therapies that will avoid the necessity of later endotracheal intubation.
First described as a treatment for pulmonary edema in 1935 [29], CPAP was later shown to decrease the need for intubation with a non-significant trend toward decreased in-hospital mortality [30]. Since then multiple clinical trials based in the ED have also investigated the role of CPAP and NIV [31,32]. Multiple meta analysis and systematic reviews [33-35] as well as Cochrane reviews [36,37] eventually established NIV and CPAP as viable treatments for acute pulmonary edema with significant reductions in hospital mortality and the need for intubation. The number needed to treat is 13 and 8 respectively.
Patients not requiring NIV or intubation will likely benefit from oxygen administration. Oxygen should be administered to maintain an O2 saturation of greater than 95% but is not required or recommended if the patient is not hypoxic.
Vasodilators and diuretics are the most important medical therapies that can be given in acute decompensated HF. In flash pulmonary edema many patients are hypertensive on presentation and vasodilator therapy is the key component in their care. Hypertensive patients require immediate afterload reduction with vasodilator therapy. Agents such as nitrates and sodium nitroprusside are commonly used in this situation.
The nitrate of choice in the ED is nitroglycerin. Nitroglycerin can be initially administered sublingual until intravenous therapy can be established. Intravenous nitroglycerin works rapidly and is easily titratable to reach your goal blood pressure. Nitroglycerin provides rapid reduction in left ventricular filling pressures and at higher doses decreases systemic afterload. Congestive symptoms are improved and stroke volume and cardiac output are increased.
Intravenous nitroglycerin in patients with chest pain is usually started at an initial dose of 10 mcg/min and titrated upward to decrease blood pressure and improve congestive symptoms. This will not be effective in hypertensive HF patients and it is recommended to start at considerably higher doses, with rapid titration. In several studies, doses of as much as 2,000 mcg every 3 minutes have been used with the result of markedly decreased intubation rates, intensive care unit admissions, and a trend to lower mortality [38]. Doses of 120 mcg/min are typically required to significantly decrease pulmonary capillary wedge pressure [39]. For comparison purposes, sublingual nitroglycerin, typically 0.4 mg or 400 mcg, can be given routinely every 60 seconds until there is a blood pressure response or the patient’s dyspnea improves.
Nitrates are contraindicated when there has been recent use of phosphodiesterase-5 inhibitors such as sildenafil which may precipitate severe hypotension. Nitrates should not be used when the patient presents initially with hypotension. Alternative to nitroglycerin include sodium nitroprusside and nesiritide. Sodium nitroprusside is also highly effective in decreasing blood pressure rapidly. It is titratable and can quickly bring down blood pressure. Sodium nitropruside has the adverse effect of accumulation of cyanide derivatives so that prolonged therapy is not recommended. Nesiritide has been shown to be not worse than other vasodilators and may also be considered as an alternative to nitroglycerin [40,41].
PATIENTS WITHOUT FLASH PULMONARY EDEMA
In patients without flash pulmonary edema, diuretics are the mainstay of ADHF treatment. A long standing consensus of experts give a class I recommendation and diuretics are an essential component of treatment [7]. In 2011 the DOSE (Diuretic Strategies in Patients with ADHF study) trial investigated optimum dosing strategies for diuretics in hospitalized HF patients. They demonstrated no differences between bolus or continuous infusion and no difference between high and low dose strategies. This multicenter trial enrolled 308 patients in a prospective, double blind, randomized investigation evaluating the patients global assessment of symptoms and change in serum creatinine from baseline. There was a trend toward greater improvement in the high dose furosemide group, as well as greater diuresis, although these effects were associated with a transient worsening of renal function [42]. Diuretics should be administered in an intravenous dose equal to 1–2.5 times the patients usual daily po dose. In diuretic naive patients, typical dosing would be to start furosemide at 40 mg or bumetanide at 1 mg, intravenous push. Subsequent dosing can then be adjusted according to urine output.
Timing of vasoactives, defined as an intravenous medication used to alter hemodynamics (e.g., nitroglycerin, nesiritide, dopamine, dobutamine, etc.) and potential treatment delay is also an issue. In patients ultimately treated with vasoactive agents, the sooner they received them the better were the outcomes for the patient. In several studies, delayed administration of vasoactives is associated with increased mortality, especially in patients with high BNP [43,44]. Morphine should not be used in ADHF. While there is historical precedent that morphine may be of value, no reasonable data exists to support its use. In fact, data from over 60,000 patients in the ADHERE (Acute Decompensated Heart Failure National Registry) found an associated increase in intensive care unit admissions and mortality associated with morphine use [45].
PATIENTS PRESENTING WITH HYPOTENSION
Patients that present with hypotension create a challenge in the management of ADHF. Hypotension is a poor prognostic sign in acute HF and the use of inotropes has not been shown to decrease mortality in patients that present with ADHF and hypotension unless it is as a bridge to mechanical therapy (e.g., left ventricular assist device) [46-48]. This is likely due to the increased arrythmogenic effects of most inotropes. Despite the disheartening data, temporary inotropic support is recommended for patients with cardiogenic shock and low blood pressure and depressed cardiac output to maintain systemic perfusion and prevent end organ damage [7]. The lowest possible dose should be used to limit arrythmogenic effects. Inotropic support may also be recommended as bridge therapy for patients who are candidates for cardiac transplantation or mechanical circulatory support [49-51]. Dopamine, dobutamine or milrinone are the common inotrope choices available in the USA, although none have proven superiority over another.
Inotropic agents, however, should not be used in the absence of specific indications in patients without documented severe systolic dysfunction, low blood pressure, impaired perfusion and evidence of significantly decreased cardiac output. Recent ACCF/AHA (American College of Cardiology Foundation/American Heart Association) guidelines give a class III recommendation (i.e., harmful) [7].
PATIENTS WITH ACUTE CORONARY SYNDROME AND HEART FAILURE
Patients presenting with ST elevation myocardial infarction and signs of congestive HF require emergent coronary angiography and revascularization. In addition in patients with angina and suitable coronary anatomy, especially significant left main or equivalent stenosis, percutaneous coronary intervention and/or coronary artery bypass grafting is also indicated. And in patients with mild to moderate systolic dysfunction coronary artery bypass grafting is reasonable to improve survival when there is significant multivessel or left anterior descending coronary artery stenosis when viable myocardium remains.
DISCHARGE vs. ADMISSION
While the overall goals of therapy are to restore normal oxygenation and improve symptoms while optimizing the patient’s volume status, it is also important to identify etiology and precipitating factors that have contributed to decompensation and hospitalization so that they may be addressed to minimize risk of rehospitalization. Risk of re-hospitalization is high in patients that did not receive adequate diuresis or fluid removal during their initial hospitalization [52].
Patients require admission for severe decompensation associated with flash pulmonary edema, hypotension, worsening renal failure or altered mentation. Patients with severe dyspnea or dyspnea at rest, tachypnea or hypoxia (pulse oximetry less than 90%) should also be admitted until symptoms improve. Patients with documented arrhythmia or high suspicion for acute coronary syndrome should also be admitted for further evaluation.
Admission should be highly considered for worsening congestion or significant volume overload felt to be, in the clinician’s opinion, not amenable to outpatient diuresis. Patients are also commonly admitted for new or previously undiagnosed HF and recurrent firing of internal defibrillator if present, as well as electrolyte disturbance or other associated comorbidities. Table 3 lists some of the more common admission criteria for ADHF.
Admission to telemetry to monitor for potential arrhythmia is recommended for 24 to 48 hours. HF guidelines recommend daily electrolyte, blood urea nitrogen and creatinine measurements during active diuretic use and titration. Thrombosis and thromboembolism prophylaxis is also recommended for patients hospitalized with HF [7].
Select patients may be admitted to the ED observation unit. Observation unit patients usually have a previous history of HF, without significant comorbidities to complicate their care and acceptable vital signs. Patients admitted to an ED observation unit have demonstrated readmission rates not higher than admitted patients with fewer bed days amongst discharged patients [53].
CONCLUSION
Patients presenting to the ED with ADHF should be evaluated and treated rapidly to ensure the best possible outcomes. The diagnosis should be made as soon as possible and therapy initiated. Flash pulmonary edema should be treated expeditiously and patients in severe respiratory distress will benefit from NIV as well as vasodilator therapy. Diuretics form the mainstay of treatment for the majority of patients with ADHF and the majority of symptomatic patients should be admitted for diuresis, decongestion and monitoring.