INTRODUCTION
Invasive mechanical ventilation (IMV) is required in approximately 3% of US hospital admissions [1]. This review will briefly discuss the use of inhaled pulmonary vasodilators (IPVs) in patients with refractory hypoxemia and provide tips for the initial administration and management.
REFRACTORY HYPOXEMIA: SHUNT AND SHOCK
Patients on IMV with persistent hypoxemia despite supplemental oxygen (FiO2) usually have significant shunt physiology and/or low cardiac output.
In shunts, venous blood passes from the right to left ventricle and then systemic circulation without participating in gas exchange. The degree of shunt fractionŌĆö(1-SaO2)/(1-ScvO2)ŌĆöwill determine the magnitude of hypoxemia [2]. Increasing FiO2 may partially improve oxygen saturation (SaO2) but in severe shunt FiO2 will not maintain acceptable SaO2. Shunts can be pulmonary or cardiac. Pulmonary shunts can be secondary to cardiogenic or non-cardiogenic pulmonary edema (acute respiratory distress syndrome, ARDS), pneumonia, hemoptysis, atelectasis or other conditions that obstruct alveoli. Cardiac shunts occur when high right ventricle (RV) afterload causes blood to flow abnormally through a patent foramen ovaleŌĆöa phenomena increasingly recognized in IMV with ARDS [3].
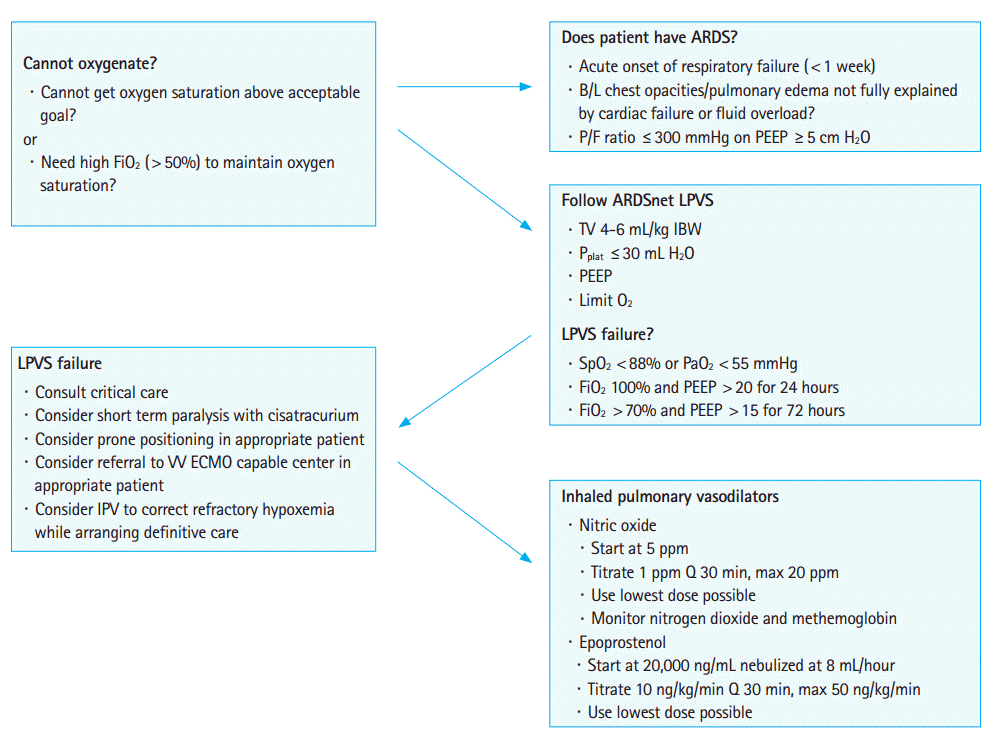
All IMV patients should be treated with a lung protective ventilation strategy [4]. Oxygen toxicity can be prevented by titrating FiO2 as low as feasible to maintain an acceptable SaO2. positive end expiratory pressure (PEEP) is used to recruit alveoli and decrease shunt fraction and can be titrated based on the ARDSnet protocol [5]. It is a validated, evidence based and easy to follow strategy. Excessive PEEP can be detrimental [6,7] and there are conditions where PEEP can make oxygenation worseŌĆöunilateral pulmonary shunts and cardiogenic shunts are such examples [3,6]. IPV and prone positioning (PP) may be better options in these scenarios situations [7].
The Berlin definition of ARDS is as follows [8]: new or worsening respiratory symptoms within 1 week of symptom onset; bilateral opacities on chest imaging not fully explained by effusions, atelectasis or nodules; respiratory failure from lung edema not fully explained by cardiac failure or fluid overload; and finally oxygenation impairment. The degree of oxygenation impairment is defined by the following PaO2/FiO2 ratios: mild, 300ŌĆō201 mmHg; moderate, 200ŌĆō101 mmHg; severe, Ōēż100 mmHg.
ARDS treatment should follow the ARDSNet low tidal volume protocol [4,5]. Patients with severe ARDS and refractory hypoxemia might be candidates for IPV and other adjunctive treatments [4,6,9].
Refractory hypoxemia is an inability to maintain acceptable oxygen saturation or an ability to maintain acceptable oxygen saturation only with unsafe ventilatory pressures or FiO2 [9]. A plateau pressure >30ŌĆō35 mm H2O (depending on abdominal and chest wall compliance) is considered unsafe [4,5,9]. Prolonged exposure to FiO2>0.6 is considered by many experts to increase the risk of oxygen toxicity [4,10].
FiO2>0.6 is considered by many [10] to increase the risk of oxygen toxicity.
IPV act as selective pulmonary vasodilators [11]. At appropriate dosage IPV selectively dilate alveolar blood vessels with good ventilation. IPV do not reach blood vessels in lung regions with poor ventilation. This reduces shunt fraction by improving blood flow to lung units with good ventilation-/perfusion ratios, decreasing blood flow through shunts and improving oxygenation [11]. In addition, IPV decrease pulmonary artery pressure and may be beneficial in respiratory failure associated with high RV afterload (pulmonary embolism, cardiogenic shock, RV infarction, right to left shunt in patients with patent foramen ovale, and certain subsets of ARDS).
INHALED PULMONARY VASODILATORS: WHEN AND HOW TO USE
For the Emergency Medicine provider, an inability to maintain an acceptable SaO2 despite appropriate PEEP and FiO2 and safe PPlat <30ŌĆō35 mm H2O constitutes refractory hypoxemia. Salvage ventilatory strategies and adjunctive techniques should be considered [9]. There are multiple different therapies for refractory hypoxemia with varying evidence and clinical benefit (Fig. 1) [4,6,8,9]. IPV have not been associated with a mortality benefit but have shown improved oxygenation and may serve as a temporary bridge to more resource intensive therapies such as PP and extracorporeal membrane oxygenation (ECMO) [9]. PP and ECMO have shown mortality benefit but require additional resources and expertise that may not immediately be available in the emergency department or indicated for certain patients. IPV may offer a good option for temporary stabilization and management of patientŌĆÖs with refractory hypoxemia [9,11]. The two main IPV are inhaled inhaled nitric oxide (INO) and inhaled prostacyclin (IP). INO has been studied more extensively than IP [11,12]. Epoprostenol has been the most frequently studied IP [11,12].
INO is initiated at 5 parts per million (PPM) and titrated 1 PPM Q 30 minutes to 20 PPM. The maximal response is usually seen at 10 PPM [11]. The effect should be immediate. If oxygenation does not initially improve discontinue INO [11]. Certain studies have suggested a higher incidence of oxygenation failure in septic shock patients on systemic vasopressors [11]. The toxic metabolites nitrogen dioxide (monitored by the INO regulator device) and methemoglobin (requires blood tests) should be watched closely. INO is generally accepted to be safe although some studies suggest an increased incidence of kidney injury and need for renal replacement therapy [11]. The lowest possible dose of INO should be used because tachyphylaxis will develop [9,11]. As there is no mortality benefit for INO, it has a relatively short time span of effectiveness (24ŌĆō96 hours), and is expensive (approximately 3,000 US dollar/day) it should only be considered short term therapy to bridge to other treatment strategies for refractory hypoxemia [8,10].
Epoprostenol is nebulized and the starting dose is 20,000-50,000 ng/mL/min [6,11]. The maximum dose is 50,000 ng/mL/min and should not be titrated faster than 10 ng/kg/min Q 30 minutes [6,11]. IP has a longer half-life than INO so there is a risk of systemic hypotension from nonselective vasodilation [11,12]. IP also can inhibit platelet function so caution should be used in patients with pulmonary hemorrhage and other conditions associated with life threatening bleeding [11]. IP is less expensive than INO ($275/day versus $3,000/day) [11].
Despite improvements in oxygenation seen with IPV, neither medication is US Food and Drug Administration approved for treatment of hypoxemia or associated with improved mortality. However, if faced with refractory hypoxemia or acute RV failure, IPV can be considered as salvage therapy while planning other interventions [6,9].