INTRODUCTION
Delayed neuropsychological sequelae (DNS) after carbon monoxide (CO) poisoning generate a myriad of neurological and psychological symptoms. The incidence of DNS has been reported to be up to 46%, and DNS remain a largely mysterious syndrome from a clinical perspective [1]. The definition of DNS encompasses a wide range of neuropsychological symptoms, including apathy, disorientation, amnesia, mutism, urinary incontinence, gait disturbance, anxiety, psychosis, depression, and Parkinsonism. It may occur within several days to several weeks following a disappearance of signs and symptoms of acute CO poisoning.
Insults to neural organs and tissues caused by CO toxicity account for several pathophysiologic factors as follows: 1) hypoxic stress, which mainly occurs when CO interferes with the binding of oxygen to hemoglobin; 2) oxidative stress caused by free radicals; 3) lipid peroxidation, which results in demyelination of white matter in neural tissue; and 4) catecholamine crisis accompanied by epinephrine release during physiological stress. These injury mechanisms may also contribute to the development of DNS [2].
Non-fire related CO poisoning is the leading cause of poisoning-related deaths in the US and is responsible for up to 50,000 emergency department visits and 1,200 deaths per year [3]. In South Korea, the number of incidents of CO poisoning has recently been increasing at a rapid pace because inhaling CO gas has emerged as a common method of suicide [4]. Those who survive CO poisoning are at high risk for neurological problems such as DNS, even after treatment of the acute condition [5]. However, epidemiologic data such as the incidence, time of development, and duration of DNS vary widely in the literature due to the lack of established diagnostic criteria that are universally accepted [6-10]. Previous studies have reported the prevalence of DNS, but they are largely limited due to small sample sizes and variations in study design. In this study, we aimed to analyze the nationwide prevalence of neurological disorders among survivors after CO intoxication using claims data from the Health Insurance Review and Assessment Service (HIRA) in South Korea.
METHODS
Ethics statement
This was a retrospective, nationwide, observational study. The institutional review board of the Ajou University Medical Center reviewed and approved the study protocols (AJIRB-MED-EXP-19-189). The requirement for informed consent was waived because all data were de-identified.
Data source and study setting
The National Health Insurance in South Korea is a public medical insurance system that provides health coverage for more than 99% of the nation’s population. The HIRA data in the National Health Insurance system include patient demographics such as sex, age, residential area, and clinical details such as diagnosis, procedure, and prescription, which can be derived from payment claims generated during outpatient visits or inpatient admissions to medical institutions. This national cohort was abstracted from the HIRA data.
Patients were included if they had been initially diagnosed with CO poisoning between January 1, 2012 and December 31, 2018. To ensure the history of the population and to remove the bias caused by the history of CO poisoning, we only included patients with a diagnosis of CO poisoning after January 1, 2012. Because we focused on the effect of CO poisoning on newly developed nervous system diseases, we excluded patients with a history of nervous system diseases before January 1, 2012 to remove the bias caused by the recurrence of already diagnosed nervous system diseases. Additionally, we excluded patients that had been newly diagnosed with nervous system diseases before the diagnosis of CO poisoning from 2012 to 2018 (Fig. 1). The diagnosis of CO poisoning (code T58) was confirmed regarding the Korean Classification of Disease 6th edition, which is a modified version of the International Classification of Diseases 10th edition designed to fit the Korean health system. We also used the G00 to G99 diagnostic codes which correspond to all nervous system diseases. These diseases were identified using the main or minor disease codes in the claims data generated during the inpatient or outpatient visits following the diagnosis of CO poisoning. The diagnosis dates of CO poisoning and nervous system disease were extracted from the attribute of the initiation date of medical care marked as ‘RECU_FR_DD’ in 200 tables of the HIRA dataset.
Statistical analysis
Descriptive statistics were used to estimate the frequency of nervous system diseases according to the diagnostic codes of the initial hospital visit. Frequently occurred nervous system diseases after CO poisoning were listed and analyzed depending on whether the patient had been admitted to the intensive care unit (ICU) due to CO poisoning or not. To compare the frequency and mean time of the occurrences of common nervous system diseases for two distinct groups (patients admitted vs. not admitted to the ICU), Pearson chi-squared test and independent samples t-tests were performed. The occurrence of nervous system diseases after CO poisoning was estimated using the Kaplan-Meier method. For statistical comparison between the ICU admitted and non-admitted groups, the log-rank test was performed. During the observation period after CO poisoning, the cases claimed as ‘4: Death’ in the attribute of results of medical care marked as ‘DGRSLT_TP_CD’ in the 200 tables of the HIRA dataset were censored. Additionally, the standardized incidence ratios (SIRs) and their corresponding 95% confidence intervals (CIs) were used to assess the relative risk of frequent nervous system disease in survivors after CO poisoning as compared with the general population based on previous research on each nervous system disease [11-15]. In this study, P-values less than 0.05 were considered to be statistically significant. All statistical analyses were performed using R ver. 3.0.2 (R Development Core Team, Vienna, Austria). The occurrence of nervous system diseases after CO poisoning was estimated using the Kaplan-Meier method. For statistical comparison between the ICU admitted and non-admitted groups, the log-rank test was performed.
RESULTS
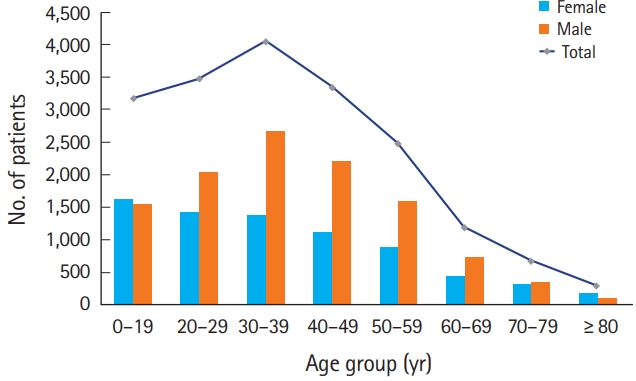
Among the total of 21,711 patients, 18,720 patients (86.2%) were diagnosed with nervous system diseases after CO poisoning from 2012 to 2018. The number of patients with nervous system diseases is shown according to age and sex in Fig. 2. The mean age at the time of diagnosis with nervous system disease after CO poisoning was 44.36 (±17.32) years; there were 7,415 female patients (39.61%). The number of patients peaked in the age group of 30 to 39 years. Overall, except for the age group of younger than 19 years and the age group of greater than 80 years, there were more male patients than female patients.
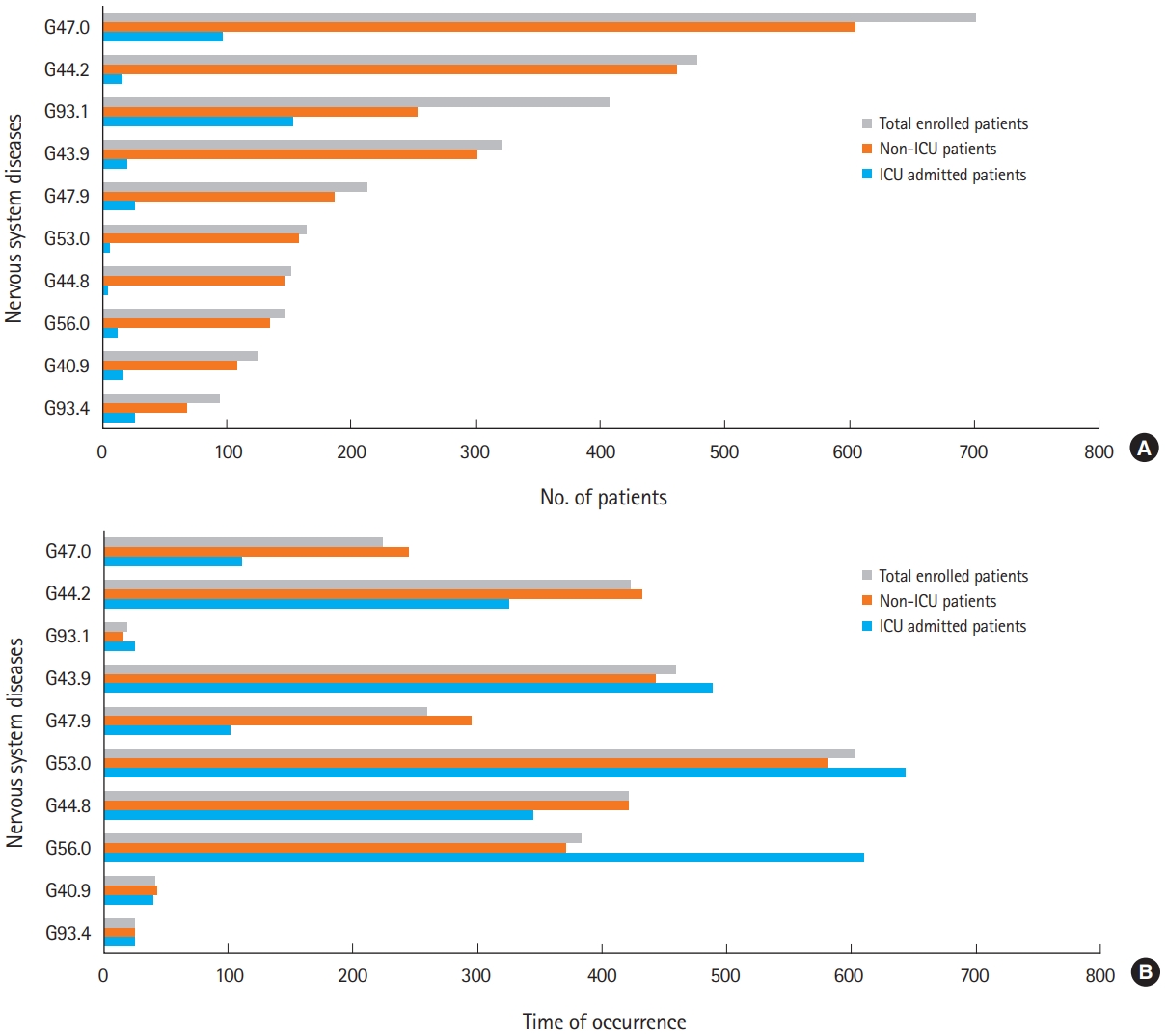
Among the 18,720 patients with nervous system diseases after CO poisoning, 1,391 patients (7.43%) were admitted to the ICU during the initial event of CO poisoning. The most common nervous system diseases after CO poisoning were disorders of initiating and maintaining sleep, G47.0 (n=701, 3.74%), followed by tension-type headache, G44.2 (n=477, 2.55%), anoxic brain injury, G93.1 (n=406, 2.17%), migraine, unspecified, G43.9 (n=321, 1.71%), and sleep disorder, G47.9 (n=212, 1.13%). The pattern of occurrence for nervous system diseases in the patients who had not been admitted to the ICU was similar to that in all patients. On the other hand, in the patients who had been admitted to the ICU, anoxic brain injury, G93.1, was the most common (n=153, 8.84%), followed by disorders of initiation and maintaining sleep, G47.0 (n=97, 7.00%) and encephalopathy, unspecified, G93.4 (n=26, 1.87%) (Fig. 3A and Table 1).
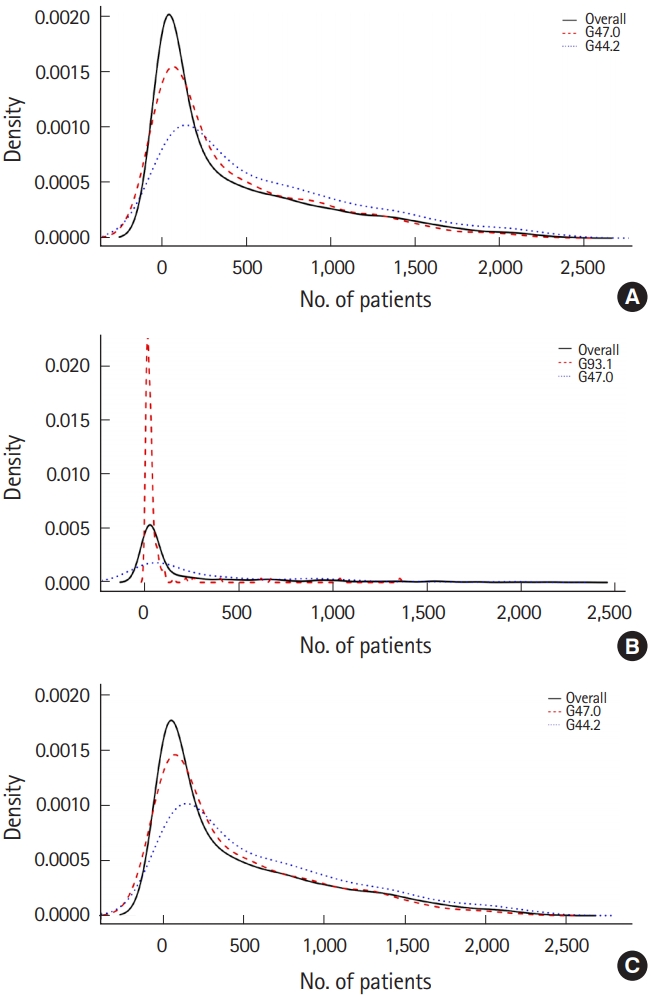
The median time to diagnosis of nervous system disease after CO poisoning differed according to whether patients were admitted to the ICU or not (P<0.05) (Fig. 3B and Table 2). The median time of occurrence for nervous system diseases after CO poisoning, e.g., other specified headache syndromes, G44.8, unspecified sleep disorder, G47.9, tension-type headache, G44.2, and sleep initiation and maintaining disorders G47.0 was shorter for the patients who had been admitted to the ICU than those who had not. The median time of occurrence for carpal tunnel syndrome, G56.0, post-zoster neuralgia, G53.0, migraine, unspecified, G43.9, and anoxic brain injury, G93.1, after CO poisoning was longer in the patients who had been admitted to the ICU compared to those who had not. The three most common nervous system diseases and their frequency density are shown in Fig. 4. Approximately 50% of the nervous system diseases were diagnosed within the first year after CO poisoning. The median follow-up time for patients who had experienced CO poisoning was 209 days (range, 1 to 2,413 days).
ICU admission for the initial event of CO poisoning was significantly associated with the development of a nervous system disease after CO poisoning. The cumulative hazard ratio for nervous system diseases in patients admitted to the ICU was 2.25 (95% CI, 2.07–2.44, P<0.05) (Fig. 5).
Patients poisoned with CO had disorders of initiating and maintaining sleep, G47.0 (SIR, 1.61; 95% CI, 1.51–1.71), tension-type headache, G44.2 (SIR, 2.41; 95% CI, 2.23–2.61), anoxic brain injury, G93.1 (SIR, 58.76; 95% CI, 53.95–63.88), post-zoster neuralgia, G53.0 (SIR, 1.94; 95% CI, 1.70–2.20), and demonstrated higher SIRs in those disorders as compared with general population. Patients who were admitted to the ICU were at risk of developing disorders of initiating and maintaining sleep, G47.0 (SIR, 3.00; 95% CI, 2.56–3.50) and anoxic brain injury, G93.1 (SIR, 290.44; 95% CI, 251.76–333.37). Patients who were not admitted to the ICU were at high risk of developing disorders of initiating and maintaining sleep, G47.0 (SIR, 1.50; 95% CI, 1.41–1.60), tensiontype headache, G44.2 (SIR, 2.49; 95% CI, 2.29–2.69), anoxic brain injury, G93.1 (SIR, 40.16; 95% CI, 36.05–44.61), and post-zoster neuralgia, G53.0 (SIR, 2.00; 95% CI, 1.74–2.28). The SIR of disorders of initiating and maintaining sleep, G47.0, and anoxic brain injury, G93.1, were relatively higher in patients admitted to the ICU than those who were not (Table 3).
DISCUSSION
The occurrence of DNS after CO poisoning is a significant prognostic problem. However, evaluating its medical impact and social burden is a challenge due to the lack of established, universally accepted diagnostic criteria. Therefore, we conducted a nationwide longitudinal study on nervous system diseases after CO poisoning using claims data provided by HIRA. Specifically, we showed the incidence types and patterns of nervous system diseases in survivors of CO poisoning. Although the risk of occurrence of nervous system disease increased in CO poisoned patients compared with the general population and the hazard ratios were higher in ICU admitted patients than those who had not, the types of those diseases were different between the patients who had been admitted to the ICU and those who had not.
This study highlights the need for a systematic diagnostic tool for DNS occurring after CO poisoning that can be used for assessing the severity of neurological status and predicting prognosis. In this study, common nervous system diseases after CO poisoning ranged from mild forms of neurological sequelae (e.g., tension-type headache and disorders of initiating and maintaining sleep) to severe forms (e.g., tetraplegia and anoxic brain injury). The mild forms of neurological sequelae such as headache or disorders of initiating and maintaining sleep may not be typical clinical characteristics of DNS which were provoked in patients after CO poisoning, although they are likely to affect patients’ quality of life because the associated symptoms can be controlled with conservative care; further, it is much less likely that patients will experience permanent neurological sequelae [16-18]. The incidence of nervous system disease in this study was approximately 86% (18,720 out of 21,711), which is much greater than in any other studies to date [6-10]. Thus, including the diagnoses of DNS with these mild forms of neurological problems may not be much of value. Current diagnostic definitions are not specific nor systematic enough to be used for estimation of the current neurologic status or to predict the prognosis of the disease. Therefore, the establishment of clinically significant diagnostic criteria is imperative for the severity of neurological problems to be determined; alternatively, DNS can be divided into either a simple or a complicated form based on the prognosis of neurological diseases after CO poisoning.
From a therapeutic point of view, active neuroprotective measures may be necessary for patients admitted to the ICU after CO poisoning because these patients are at a greater risk for developing nervous system diseases than patients who were not admitted to the ICU. Although the prognostic factors that predict the occurrence of DNS after CO poisoning have not been elucidated, several previous studies have reported evidence of an association between a poor clinical status in patients with severe CO poisoning and the development of DNS after CO poisoning [1,19-21]. Consistent with these results, we found that the patients who received care in the ICU due to severe CO poisoning showed a significantly higher risk of developing nervous system disease later on compared with those who did not received care in the ICU. To reduce the occurrence of neurological sequelae in critically-ill patients, neuroprotective methods such as therapeutic temperature management, hyperbaric oxygen therapy, anti-inflammatory agents, and antioxidant drugs could be administered to patients in the ICU after severe CO poisoning, although the efficacy and safety of these methods should be further clarified [2,9,22–24].
However, considering the SIR of common neurological diseases according to the history of admission to the ICU, the risks of neurological diseases after CO poisoning were not always higher in the ICU admitted group than in the non-ICU admitted group. Because of the withdrawal of sedatives or analgesics after the period of intensive treatment (e.g., mechanical ventilation and invasive monitoring in the ICU), the risk of disorders of initiating and maintaining sleep in the ICU admitted group might be higher [25]. In addition, anoxic brain injury is also a typical sequela after CO poisoning, along with cardiac arrest, and it is assumed that anoxic brain injury could occur frequently in patients who are in a severe enough condition to be admitted to the ICU after CO poisoning [13]. Nevertheless, in patients who were not admitted to the ICU, the risk of developing tension-type headache and post-zoster neuralgia was higher than in the patient group admitted to the ICU, as well as in the general population. This indicates that different pathophysiology may occur in patients depending on the degree of CO poisoning; this assumption needs to be investigated in the future.
Our study has several limitations. First, we confirmed the diagnoses of CO poisoning and nervous system diseases based on the claims data extracted from the HIRA, but the HIRA data only provides the disease codes and demographic information. Thus, there was no data available on the details of clinical signs and symptoms, intentionality, co-exposure materials, laboratory data, imaging results, or social histories such as smoking, alcohol, and family history. And, because of lack of clinical information on exposure to risk factors of nervous system diseases during the period between CO poisoning and diagnosis of nervous system diseases, it is difficult to determine the causality of CO poisoning and nervous system diseases. A national cohort study that combines the HIRA claims data with clinical information is necessary.
In addition, the HIRA data lacked evidence of lucid intervals from the CO poisoning event to the occurrence of nervous system diseases. Our findings on the prevalence of nervous system diseases after CO poisoning may not correspond to the incidence status of DNS after CO poisoning. In addition, considering that DNS generally occurs within several days to several weeks after the CO poisoning event, the reported occurrences of nervous system diseases in our study, which counted cases of nervous system diseases that occurred several years after CO poisoning, could have overestimated the development of DNS after CO poisoning. Further, the development status of DNS may also have been underestimated in the situation that the patient was unable to visit the hospital immediately and visited the hospital with neurological signs and symptoms later after the CO poisoning event. Lastly, the HIRA claims data do not provide information about the severity of CO poisoning; thus, ICU admission only may not be directly related to the severity of CO poisoning.
Moreover, even though we have identified the types and patterns of nervous system diseases which occur frequently after CO poisoning, because of the lack of previous study and database about statistical information on nervous system diseases that occur frequently after CO poisoning in South Korea, comparison with the control group based on data studied abroad was performed. If a validated registry program for nervous system diseases is established in South Korea in the future, further studies can be conducted regarding explaining the effects of CO poisoning on the occurrence of nervous system diseases.
In summary, in caring for CO poisoned patients, clinicians need to take into account that nervous system diseases following CO poisoning have an early peak, developing within the first year. Although active therapeutic interventions for neuroprotection for these patients admitted to the ICU after CO poisoning may help to reduce the development of nervous system diseases, because nervous system diseases in survivors of CO poisoning had different patterns of incidence depending on whether the patient had been admitted to the ICU or not, a sophisticated approach for considering neuropsychological complications in patients with CO poisoning is needed.