INTRODUCTION
Pneumatosis intestinalis (PI) is the presence of gas within the wall of the gastrointestinal tract. It is not a clinical diagnosis, but a physical or radiological finding resulting from an underlying pathological process [1]. PI is classified as primary (idiopathic) or secondary. Generally, primary PI is managed conservatively. In contrast, secondary PI could be fatal and requires surgery.
Herein, we present two patients diagnosed with PI and concurrent renal cell carcinoma (RCC), who presented with abdominal distension after anticancer chemotherapy. In addition, we discussed the treatment of PI in patients with cancer.
CASE REPORT
Case 1
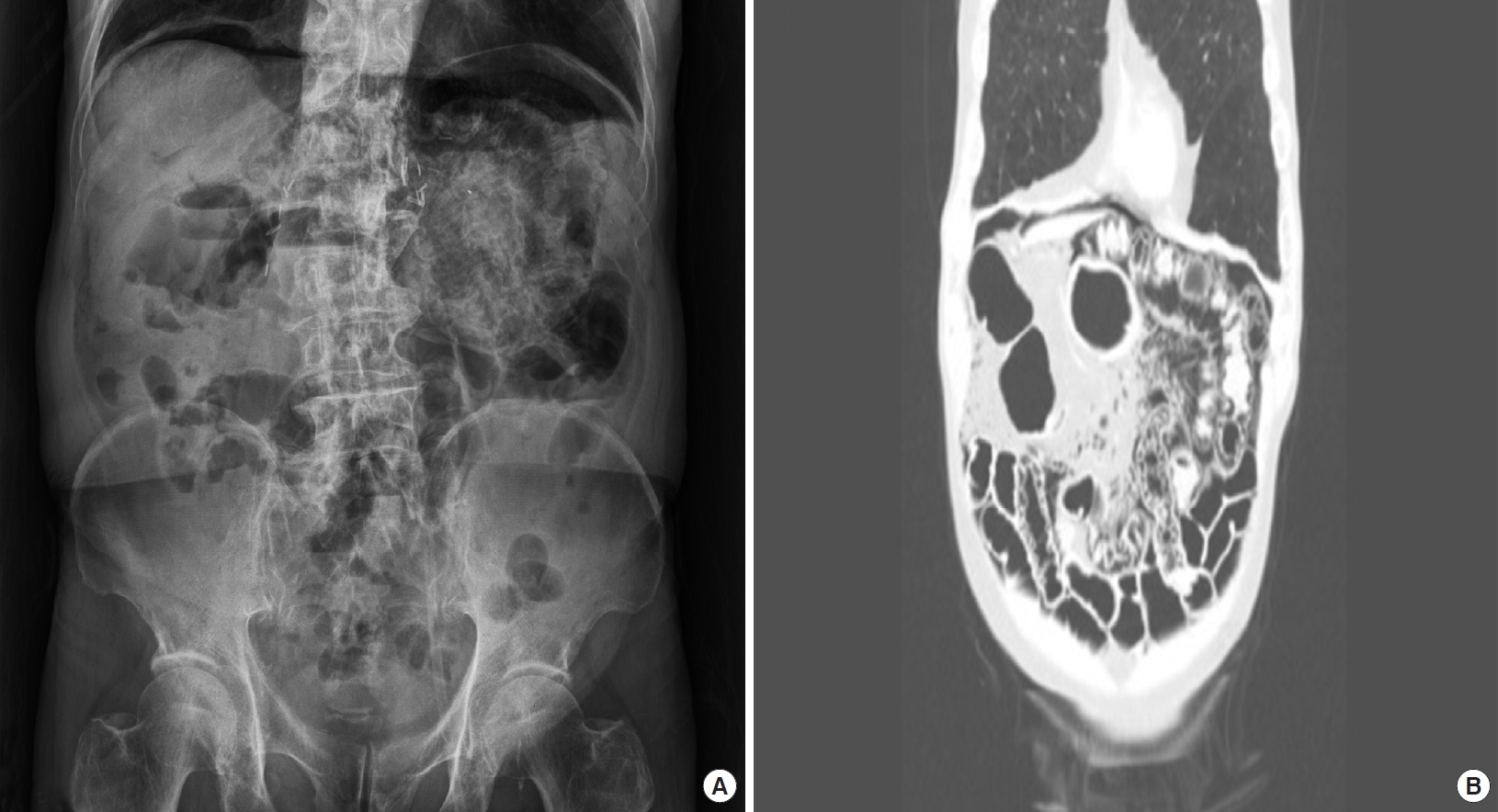
An 81-year-old male presented to our emergency department (ED) with painless post-prandial epigastric discomfort for five days. He had a 12-year history of RCC, chronic obstructive pulmonary disease, hypertension, and chronic renal failure. The patient was receiving chemotherapy for RCC. A month ago, he has taken 40 mg of methylprednisolone for chronic obstructive pulmonary disease exacerbation for 3 days, and he was on 5 mg of axitinib (Inlyta; Pfizer, New York, NY, USA) daily for the past year. His vital signs were within the normal range. Apart from elevated blood urea nitrogen (55.5 mg/dL, 9.0ŌĆō23.0 mg/L) and creatinine (1.7 mg/dL, 0.7ŌĆō1.3 mg/dL) levels, his blood tests (complete blood count with differential count, liver function tests, coagulation tests, electrolytes, and lactate level, etc.) were within normal ranges. Plain abdominal radiography and abdominal computed tomography (CT) without contrast revealed diffuse PI, predominantly in the jejunum, with a large amount of intra-abdominal free air (Fig. 1). Following a suspicion of bowel perforation, an exploratory laparotomy was performed. However, during the operation, we only found PI, which occupied 20 cm of the bowel wall 1 m away from the ileocecal valve without ischemic changes or bowel perforation (Fig. 2). On postoperative day (POD) 4, the patient started eating soft foods and did not develop any complications. On POD 8, plain abdominal radiography was performed, which showed the absence of any free air. However, the patient developed diarrhea after resuming foods. On POD 17, abdominal CT was repeated, which showed the absence of PI or pneumoperitoneum. The patient was discharged on POD 34 without any complications.
Case 2
A 63-year-old male was referred to the ED by a radiologist who found PI and pneumoperitoneum incidentally on CT during his regular check-up. He complained of a migrating discomfort in both flanks and abdominal distension, which started a month prior to this consultation. He had a medical history of RCC and hypothyroidism. The patient was receiving chemotherapy for RCC. He had been taking 40 mg of cabozantinib (Cabometyx; Exelixis, Alameda, CA, USA) daily for the past year. His initial vital signs were within the normal range. He had elevated blood urea nitrogen (27.3 mg/dL) and creatinine (1.81 mg/dL) levels. However, other blood tests including lactate level were within normal ranges. Plain abdominal radiography and abdominal CT without contrast revealed diffuse PI, predominantly in the jejunum, with free air in the perihepatic space (Fig. 3). He was hospitalized and managed conservatively. He started eating soft foods on postadmission day 3. He was discharged on postadmission day 5 without complications.
DISCUSSION
PI is caused by several underlying gastrointestinal or extra-gastrointestinal diseases, such as autoimmune diseases (e.g., scleroderma), inflammatory diseases (e.g., inflammatory bowel disease), infectious diseases (e.g., Clostridium difficile infection or human immunodeficiency virus), pulmonary diseases (e.g., chronic obstructive pulmonary disease), drugs (e.g., corticosteroids and immunosuppressive agents), and trauma (e.g., blunt abdominal trauma) [1,2]. Studies suggest that PI occurs due to disruption of the mucosal integrity. There are two major theories to explain the cause of the intramural gas [3] : (1) the bacterial theory: gas-producing bacteria translocate from the gastrointestinal lumen to the submucosal space through mucosal gaps or areas of the enhanced permeability and (2) the mechanical theory: normal gas moves from the lumen into the non-inflamed bowel wall due to disruption of the mucosal integrity.
Primary PI (15%) is asymptomatic, rare, and usually located in the colon. Individuals who are at a high risk of primary PI are males aged from 40 to 60 years [4]. Secondary PI is more common and can be fatal; it is associated with several underlying conditions, such as coronary artery disease, peripheral vascular disease, and smoking, which could lead to acute mesenteric ischemia [5]. A case series of 919 patients with PI showed that PI had a peak incidence among people in their 40s, with a male predominance (male-to-female ratio 3:1). PI affected all parts of the gastrointestinal tract: small bowel (42%), large bowel (36%), and both (22%) [6]. Patients with cancer usually receive immunosuppressive therapies, which can reduce the number of lymphocytes in the gastrointestinal wall, particularly in PeyerŌĆÖs patches, and thereby impairing the bowel defense barrier system [7]. In a case series of 84 cancer-related PI cases, the CT findings associated with clinically worrisome PI were portomesenteric venous gas (PVG), bowel dilatation, bowel wall thickening, ascites, and peri-intestinal mesenteric stranding, whereas PI confined in the colon was benign [3].
In a previous report of three PI cases, an association between PI and steroids or chemotherapeutic agents (e.g., lapatinib, capecitabine, zoledronic acid, docetaxel, etc.) was found, and all the cases were benign. Such cases can be resolved with supportive care alone and close observation [8]. The most common cause of nonsurgical pneumoperitoneum is PI [9,10]. When a cyst ruptures, it may result in a pneumoperitoneum with a spectrum of symptoms ranging from asymptomatic to acute abdominal pain. Even when it mimics secondary PI, patients with gross radiological evidence could follow a more benign clinical course [10,11]. Thus, when there are no abdominal signs of peritonitis and the patient is afebrile and has normal white blood cell counts, conservative management should be considered [11].
In case 1, the patient had received targeted anticancer therapy using axitinib. In this patient, PI was confined to the small intestines with pneumoperitoneum; he did not have abdominal pain or any evidence of bowel ischemia. Although he did not have potentially fatal symptoms, such as severe abdominal pain, acidosis, and fever, which are frequently seen in secondary PI, the general surgeon performed an exploratory laparotomy to rule out bowel perforation. After confirming the absence of bowel perforation, the surgeon concluded that the patient should be managed conservatively. Due to his immunosuppressive status, old age, and imaging results, the surgeon was skeptical of the patientŌĆÖs condition; however, conservative management and follow-up observation were successful.
Case 2 was similar to case 1. The patient was receiving targeted anticancer therapy. In case 2, the PI was confined to the small bowel with pneumoperitoneum, and the patient did not have pain. His symptoms were not worrisome. He was managed conservatively during his 5-day hospitalization.
Clinicians must carefully decide how to treat a patient with PI, especially those with cancer. In a case series of 88 patients with PI, the patientsŌĆÖ characteristics and treatments were reviewed, and a management algorithm was constructed [5]. Among the 84 patients, 70 patients were included in an exploratory series, while 14 were included in a confirmatory series. Among the 70 patients, 19 had benign disease, and six of them with PI received chemotherapy. Among them, ten patients were managed conservatively, and nine patients underwent exploratory laparoscopy. There were no signs of bowel injury, ischemia, or necrosis. A management algorithm was constructed using the results of the exploratory series. A 3-step algorithm consists of the patientsŌĆÖ stability (critically ill and unstable), radiologic findings (PI/PVG with mechanical disease or iatrogenic gastrointestinal trauma), and vascular disease score (determined by patientsŌĆÖ medical history, physical examination, lactate level, and radiologic findings; a score below 4 suggests benign PI). Seven of 14 patients in the confirmatory series had benign PI; all their vascular disease scores were below 4. One patient underwent non-therapeutic laparoscopy, and the postoperative period for all the patients was uneventful.
Furthermore, based on the management algorithm for PI and/or PVG, we can classify the PI in our patients as benign. They were not critically ill or unstable, and they had no vascular obstructions or iatrogenic GI trauma. In addition, the vascular disease score was 2.5 and 1.5, respectively.
The management of patients with PI remains controversial. In patients with PI and concurrent pneumoperitoneum, clinicians cannot be certain of the presence of bowel perforation. In addition, surgical exploration is not a simple procedure wherein a patient can recover completely several hours after the operation. For this reason, clinicians should thoroughly review PI cases, apply a management algorithm to patients with PI, and then carefully select a treatment option. If the patient has benign PI, clinicians should observe their patients closely. If the patient has secondary PI, surgical management should be provided immediately. Non-surgical treatment of benign PI can improve patientsŌĆÖ health and shorten their hospitalization time. Physicians should consider primary PI associated with anticancer chemotherapy when a patient presents with vague symptoms at the emergency department. Careful consideration of the patientŌĆÖs history, thorough examination, and assessment of diagnostic clues is mandatory.