Addressing overuse in emergency medicine: evidence of a role for greater patient engagement
Article information
Abstract
Overuse of health care refers to tests, treatments, and even health care settings when used in circumstances where they are unlikely to help. Overuse is not only wasteful, it threatens patient safety by exposing patients to a greater chance of harm than benefit. It is a widespread problem and has proved resistant to change. Overuse of diagnostic testing is a particular problem in emergency medicine. Emergency physicians cite fear of missing a diagnosis, fear of law suits, and perceived patient expectations as key contributors. However, physicians’ assumptions about what patients expect are often wrong, and overlook two of patients’ most consistently voiced priorities: communication and empathy. Evidence indicates that patients who are more fully informed and engaged in their care often opt for less aggressive approaches. Shared decision making refers to (1) providing balanced information so that patients understand their options and the trade-offs involved, (2) encouraging them to voice their preferences and values, and (3) engaging them—to the extent appropriate or desired—in decision making. By adopting this approach to discretionary decision making, physicians are better positioned to address patients’ concerns without the use of tests and treatments patients neither need nor value.
INTRODUCTION
A significant portion of the medical care patients receive is of little or no benefit, or is as likely to harm as help. According to a 2012 Institute of Medicine report, unnecessary services, commonly referred to as overuse, comprise the single largest source of waste in US health care—waste accounting for as much as 30 percent of all health care spending [1]. The implications of overuse for patient safety are equally troubling. Tests, treatments, and even intensive health care settings, used in circumstances where they are unlikely to help, expose patients to unnecessary risk. This may involve direct harms, such as a Clostridium difficile infection resulting from antibiotics prescribed for a viral infection; or indirect harms, as when a computed tomography (CT) scan performed for low-risk headache leads to false positive or clinically unimportant findings, generating anxiety for the patient and the inconvenience and risk of further testing or treatment.
Addressing overuse has been identified as a national priority for US health care reform [2]. Recent years have seen widespread calls for change [3,4], high-profile efforts to raise physician awareness such as the Choosing Wisely initiative [5], dedicated series in leading medical journals (JAMA Internal Medicine’s “Less is more,” the BMJ’s “Too much medicine,” the Lancet’s “Right care”), and a rapidly expanding research literature on the topic [6]. Yet in practice, progress has been slow [7,8], even as physicians themselves acknowledge the problem [9,10]. This review will examine overuse in the context of emergency medicine (EM), and emerging evidence of an underutilized mechanism for reducing it.
EXAMPLES OF OVERUSE IN EM
Overuse takes different forms in different specialties. Unnecessary invasive procedures predominate in some; medications and screening tests in others. In EM, overuse of diagnostic testing is the problem [11]. In a 2015 survey, over 85% of 435 emergency physicians (EPs) felt that too many diagnostic imaging tests were being ordered in their own departments, and 97% felt that at least some (mean 22%) of the advanced imaging studies they themselves ordered were not medically justified [9]. In 2014, the American College of Emergency Physicians, a latecomer to Choosing Wisely, produced a list of 10 recognized sources of overuse in EM to avoid (Table 1); advanced imaging, mainly CT, accounts for half of these.
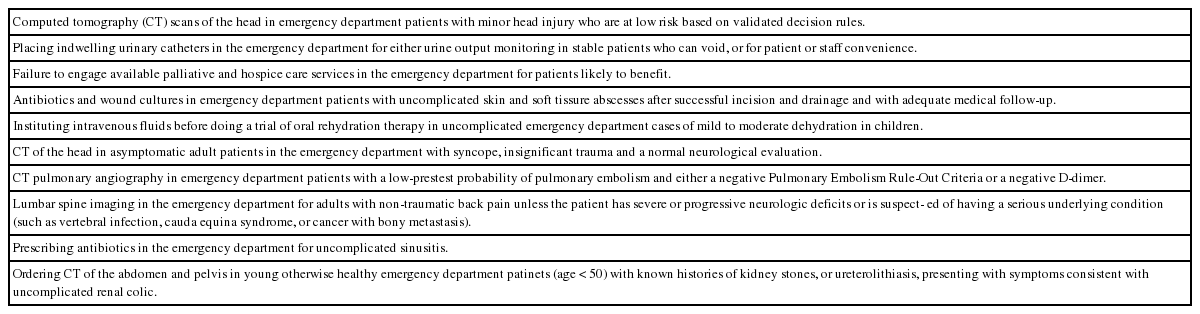
Common forms of overuse in emergency medicine as identified by American College of Emergency Physicians for the Choosing Wisely initiative
Much of the evidence of CT overuse comes from observations that, despite steep increases in CT use for a variety of indications such as trauma-related conditions, pulmonary embolus, and respiratory infections, no corresponding improvement in clinically important disease detection has been seen [12-14]. Other studies have shown high rates of guideline-discordant CT use, including a third of CTs ordered for minor head injury13 and a similar proportion of those used to rule out pulmonary embolus [14]. Of note, CT ordering rates vary strikingly across EPs—as much as 8-fold, for example, in the case of abdominal CT for patients not requiring admission [15].
Additional examples of overtesting and its consequences in EM include the routine initiation of, or referral for, cardiac work-ups for low or very low-risk chest pain patients, which increase cardiac catheterization and revascularization rates without improving outcomes [16]; the use of D-dimer in patients at very low risk of pulmonary embolus, leading to excess imaging [17]; and the ordering of urinalysis for asymptomatic patients, risking inappropriate antibiotic treatment [18]. Common instances of primary overtreatment in EM include prescribing antibiotics for acute bronchitis [19], and placing medically unnecessary urinary catheters [20].
INSIGHTS INTO OVERUSE FROM STUDIES OF PRACTICE VARIATION
Research dating back to the 1970s lent valuable insights into overuse. Epidemiologist and physician John Wennberg and a colleague, tracking health care delivery patterns in Vermont and Maine, made what was at the time a surprising discovery: rates of surgical procedures varied markedly from one part of a state to another—even between neighboring districts [21]. Finding no patient factors to explain, for example, nearly four-fold differences in tonsillectomy rates among school children, they determined that “variations tend to reflect differences in the way particular individuals and groups practice medicine.”
Subsequent work by Wennberg and the Dartmouth Atlas Project showed that greater health care utilization in a given region mirrors greater concentrations of local health care resources, e.g., specialists, medical technologies and hospital beds—but is associated neither with sicker patients to begin with nor with better outcomes [22,23]. Supply-sensitive care, as such care is known, is encouraged by the combined effects of a fee-for-service, third-party payment model. But the wider insight is that substantial variations in care may be driven by factors having nothing to do with patients themselves, nor with scientific evidence, and to this extent are inconsistent with the aims of health care. An understanding of this concept is central to the study of overuse.
DRIVERS OF OVERUSE IN EM
Financial incentives, while undoubtedly behind much of the overuse in health care, are only one among many non-clinical pressures acting on physician decision making. In fact, personal financial motive is unlikely to explain most overuse in EM, given that many EPs—particularly those in the academic sector or paid on salary—are entitled at best only to indirect remuneration for individual services provided. A bigger factor, EPs say, is fear. In a 2015 survey by Kanzaria et al. [9], EPs identified fear of missing a low-probability diagnosis (69%) and fear of litigation (64%) as the leading contributors to unnecessary imaging in EM. Indeed, defensive medicine and more specifically “assurance behaviors”—ordering tests or treatments with the aim of protecting the physician against future legal action by the patient or patient’s family—are widespread among EPs [11].
Risk-aversion and intolerance for uncertainty are unsurprising, given the context in which EPs function. EM pairs high-stakes decision making with a unique set of challenges. These include lack of time (limiting the opportunity to gather a thorough history, learn potentially relevant psychosocial or other contextual factors, and establish trust), lack of an ongoing relationship between physician and patient, inconsistent access to prior medical records, and uncertain access by patients to appropriate follow-up. There is also a widespread perception among EPs that they face especially high medicolegal risk, but a 2011 Research and Development (RAND) Corporation study indicated only slightly aboveaverage claims rates, average payout rates, and below-average (both mean and median) total payouts [11].
Additional drivers of overuse cited by EPs in the survey by Kanzaria et al. [9] were perceived patient or family expectations (38%), perceived local (39%) or EM-wide (35%) standard of care, and lack of time (24%); administrative pressure and personal reimbursement were infrequently cited. Other likely contributors include the ease and speed with which tests can be obtained, especially with the advent of electronic order entry [24]; the inconsistent availability of high-quality evidence synopses; and quality metrics which track underuse but less commonly overuse [25]. Importantly, these myriad influences are offset by no comparable influences in the opposite direction (toward judicious, evidence-based care) and have therefore been called a “perfect storm” of conditions for overuse [26].
REDUCING OVERUSE
Is change possible under such circumstances? Large-scale reform of health care overuse, most believe, will require systems-level solutions, including alternative payment models that reward quality over volume; national initiatives aimed at promoting evidence-based standards and appropriate-use criteria; administrative-level efforts such as feedback to physicians on test-ordering metrics; and medical education reform. Surveyed EPs say they believe tort reform is needed above all, but studies to date have failed to show a reduction in practice intensity (e.g., imaging rates) in states which have undergone tort reform [27]. Measures to curb overuse will, in any case, take time. Meanwhile, from the standpoint of the practicing EP, it has been argued, a risk analysis will continue to favor doing more rather than less [28].
One influence on physicians’ decisions, however, though currently perceived as part of the problem, may have the potential instead to help: patients’ own preferences for care. Physicians are not only duty-bound to place patients’ interests first in making health care decisions, patients have a legal right to information about their health care options, and to weigh in on these – to refuse specific care, for example, or to request one approach over another, where more than one reasonable option exists. Engaging patients in their health care is increasingly seen, too, as an ethical mandate for physicians [29]. In addition, this may be the only means of ensuring that services provided are valued by the patient. Health care that informed patients don’t value, insofar as it is preference-sensitive (may be approached in more than one way) amounts to overuse. A growing body of research, detailed below, suggests that a simple physician-led change (greater information exchange and engagement with patients) has the potential to reduce such care.
UNTAPPED PATIENT PERSPECTIVE
Patient expectations as perceived by physicians
Both elsewhere in medicine and in EM, physicians are frequently swayed by what they perceive their patients’ (or patients’ families’) expectations to be [9,30]. Mangione-Smith et al. [31] found physician perception of parent expectations to be the sole predictor of whether antibiotics were prescribed (62% vs.7% of the time) for children with upper respiratory infections. A national survey of 1,662 physicians across a range of specialties found that 36% would order an unnecessary magnetic resonance imaging (MRI) for acute low back pain if the patient insisted [32].
But physicians are often mistaken about patient expectations and preferences, even as they try to meet them. EPs treating patients with diarrhea have been found more likely to prescribe antibiotics if they believe patients expect them, but they correctly identify expectations only a third of the time [33]. And in Mangione-Smith et al.’s study [31], antibiotic prescriptions reflected parents’ perceived expectations, but not their actual expectations. Much evidence has shown that physicians’ assumptions regarding patient priorities are frequently incorrect [34,35] and, furthermore, that their decisions on behalf of patients differ from those they would make for themselves [36].
Even when physicians are correct about patients’ expectations, they may assign them too much weight: fulfillment of expectations has not been shown to be a consistent or dominant factor in patient satisfaction. A review of 19 studies of visit-specific expectations found only a modest relationship between expectations and satisfaction [37]. A subsequent study of over 3,000 adults with acute cough found that while satisfaction was somewhat associated with whether hoped-for antibiotics were prescribed (odds ratio 0.39), 93% of patients were satisfied even though only half had received antibiotics.38 Other studies report similar findings [33,39], or no association at all between patient satisfaction and whether pre-visit expectations were met [31,40]. Importantly, while the evidence links medically unnecessary care less to patients’ demands than to physicians’ perceptions of these, such care has been found reinforce patient expectations in subsequent medical encounters [41-43].
To the extent that meeting patients’ expectations for specific tests or treatments sometimes does predict greater satisfaction, patient satisfaction can be at odds with health outcomes. In a randomized controlled trial of imaging versus no imaging for patients with low back pain, for example, patients in the imaging group were more likely to report pain at 3 months and to have consulted their physician again, yet were ultimately more often satisfied with their care [44]. In similar study comparing X-rays and MRI, back pain patients who got MRI were more satisfied despite comparable pain and disability scores and a trend toward more operations [45].
Patient expectations as reported by patients
The pursuit of patient satisfaction is no guarantee of high-quality care—nor even, where patients’ preferences are inferred and not confirmed, of satisfaction itself. But there is nevertheless much to be learned from what patients say matters to them.
Studies have overwhelmingly found the quality of physician-patient communication to be a top patient priority [19,31,40,46]. Patients rank the provision of information as highly as [47] or higher than [31] that of specific services. Provider interpersonal behavior or humanism, likewise, has been found to be a strong predictor of satisfaction among ED patients [48], empathic caring ranking higher even for patients than information in one ED study [49]. How much time patients feel the physician spent listening to them can also predict satisfaction levels [40].
Failure of physicians to deliver on these expectations is not only a common patient complaint, it is among the factors most frequently cited by patients who sue [50]. A systematic review of 14 studies found that 26% to 95% (median 52%) of patients were dissatisfied with the information they received and desired more [51]. In interviews with families who filed malpractice claims following perinatal injuries, most complained about at least one aspect of physician-patient communication, including “would not answer questions,” “would not listen,” or “attempted to mislead” them; by way of comparison, only 25% cited money as a factor [52]. Even among patients who had not sued their physicians, those whose obstetricians had frequent malpractice claims were twice as likely to complain about their care as those whose obstetricians had never been sued, and the most frequent source of complaints was physician-patient communication: feeling rushed, not being offered information, not being listened to, and perceiving a lack of concern or respect on the physician’s part [53].
Patient concerns
Patients often have specific concerns which they may or may not volunteer. Failure of physicians to identify and address these, coupled with the reflexive ordering of tests or treatments, can represent a missed opportunity to reassure through dialogue and education. For example, physicians may assume parents expect antibiotics when instead they may merely be looking for assurances that their child’s illness isn’t serious, or validation of their decision to seek medical care [54]. And for a majority of adults with upper respiratory symptoms, the goal is a diagnosis [55,56], not necessarily antibiotics [43,55].
Requests for imaging, e.g., among patients with low back pain, may reflect a desire by patients for validation of their suffering [57]. Demands driven in this way by emotional, not rational, motives may be possible to address at an interpersonal level. A study of patients with low back pain found that functional limitation and the desire to know the cause were patients’ main concerns [58]. And an ED study by Melnick et al. [49] on imaging decisions in minor head trauma found that reassurance, listening, and having their concerns addressed were the most frequently occurring themes for patients.
Additional factors that may contribute to patient requests for unnecessary care include lack of knowledge, direct-to-consumer advertising, moral hazard resulting from third-party payment, and the fear that physicians and health care facilities may ration care to cut costs. Rather than undermine the value of communication and empathy, however, these challenges underscore the importance of addressing patients’ expectations explicitly and forthrightly, providing relevant education, listening, and reassuring. Indeed, evidence exists that brief patient education can increase patient satisfaction when, for example, imaging is not recommended [41]. By comparison, diagnostic tests for symptoms with low risk of serious illness have not been consistently found to reassure patients, decrease anxiety, or resolve symptoms [59].
Patient attitudes toward health interventions
Patients frequently hold beliefs about health care that run counter to the evidence [60,61]. In particular, patients tend to believe that more care means higher-quality, better care [62], and to be biased in favor of intervention [63]. Most overestimate the benefits of health care and underestimate the harms, a large systematic review has shown [64].
But patients’ beliefs about health care are at least in part a reflection of their prior experiences with it. Intervention bias on the part of physicians is well documented [63,65]; like patients, physicians tend to assume benefits and overlook the possibility of harm [65]. In a large national survey examining decision making processes, most patients having faced a discretionary screening test decision reported being advised by their physicians to opt for the test and infrequently informed of reasons not to [66]. In audio recordings of cardiologists discussing treatment of stable coronary artery disease, stents were recommended in most cases, but harms, benefits and treatment alternatives were rarely fully discussed [67].
Evidence suggests, however, that when patients are provided with balanced information, they show less inclination to undergo interventions. In considering whether to take medication for cardiovascular disease prevention, for example, older patients have been found to be more sensitive to knowledge of adverse medication effects than to knowledge of benefits [68]. And patients with stable coronary artery disease are less likely to agree to an invasive procedure, the more elements of informed decision making are fulfilled [67].
SHARED DECISION MAKING
Taken together, the evidence reviewed above argues for medical decision making that is centered on information exchange and explicitly identifies and addresses the individual patient’s concerns and preferences. This two-way exchange between patient and physician is the essence of shared decision making (SDM).
SDM arose as a concept in the 1980s, propelled by a cultural shift in medicine toward greater patient autonomy and away from paternalism. It is by now widely endorsed. In its 2001 report, “Crossing the quality chasm: a new health system for the 21st century,” the Institute of Medicine explicitly encourages SDM, recommending that patients “be given the necessary information and the opportunity to exercise the degree of control they choose over health care decisions that affect them [69].” American College of Emergency Physicians’s code of ethics contains similar language [70]. The Affordable Health Care Act also contains a provision aimed at promoting SDM.
The following definition of SDM emerged from the 2016 Academic Emergency Medicine Consensus Conference on the topic: “A conversation between…clinician and…patient in which they figure out together what to do to address the patient’s situation [71].” SDM has been described as a continuum: how and to what extent a patient participates in decision making may vary according to the patient’s desire and ability, and to how value-laden vs. value-neutral a decision may be [72]. As an answer to the issues outlined earlier—misunderstood patient priorities, patient dissatisfaction with physician communication efforts, and intervention bias often stemming from lack of information—SDM is less about patients making decisions than about enabling them to do so, through information exchange; inviting them to, to the extent they desire; and, wherever possible, arriving at decisions which reflect their values and preferences.
SDM is no less applicable to EM than to other specialties, in all but the most immediately life-threatening situations [73], assuming (1) an awake patient able to absorb information about the medical problem and the options for approaching it, and (2) more than one reasonable option. A high level of understanding is not required, but information should be commensurate with the patient’s ability to use it and readiness to receive it. As a rule of thumb, not engaging patients in their care assumes: (1) a single best approach exists, (2) the physician knows the best approach and consistently applies it, (3) the physician is in a better position than the patient to evaluate trade-offs between approaches, and (4) the physician has a legitimate investment in the decision, i.e., has the patient’s interests in mind [74].
Evidence of benefit
Evidence of the benefits of SDM comes mostly from studies using decision aids (DAs)—visually accessible information displays in a variety of formats aimed at facilitating SDM. A large Cochrane review found that, compared with those getting usual care, patients who use DAs demonstrate greater knowledge, more accurate perception of treatment benefits and harms, and less tendency to remain passive during decision making, and they experience no greater anxiety [75]. Benefits from SDM have also been seen in the absence of DAs. For example, an observational study of older ED patients with acute musculoskeletal pain found that patients who reported greater participation in decision making were more satisfied with the discharge pain medications [76].
Importantly, informed patients encouraged to consider their preferences appear to choose more conservative options. Patients with back pain who watched a video of other patients explaining their treatment preferences were less likely to choose surgery than patients receiving only written information; 1-year outcomes were the same [77]. And in the Cochrane review, patients using DAs chose more conservative options than their physicians [75].
Studies of DAs in the EM literature support this finding. In one, ED patients with chest pain or dyspnea were presented with a hypothetical scenario involving a standardized description of the workup for possible pulmonary embolus, along with a DA describing the risks of CT in low-probability patients and of deferring imaging assuming a D-dimer less than twice the upper limit of normal; over a third of patients elected to defer imaging [78]. Two other studies involved low-risk chest pain patients. In one, the Chest Pain Choice DA, an individualized pictogram indicating risk of an adverse event, was found to safely reduce rates of admission for observation [79]. In the other, patients given a printout of their pretest probability were less likely to opt for CT, more satisfied with physician explanations, and less likely to return to the ED within7 days, with no increase in missed or delayed diagnosis [80].
SDM is far from a guarantee of less extensive testing. In fact, concerns have been raised that linking it to the hope of greater efficiency could detract from its intended focus on patients’ interests [81,82]. Few if any have suggested that SDM be employed in the interest of saving money. But addressing unnecessary care is very much in patients’ interests. SDM is the most direct way to ensure that patients aren’t subjected to preference-sensitive interventions they don’t value or, worse, are no more likely to help than to harm them.
How patients feel about SDM
Most patients favor at least some level of involvement in health care decisions. An overwhelming majority wish to be offered choices and to be asked their opinion; interest in helping make the final decision varies somewhat but is nevertheless substantial [83]. In a European study,70% of 8,000 patients had a high desire for SDM [84]. More than 90% in one US study wanted a shared role in deciding on screening and diagnostic tests [85]. In an ED study, parents of children with lacerations overwhelmingly wished to be included in medical decisions affecting their children.86 Moreover,71% of studies in or after 2000 found that most patients wanted role in decision making, compared with only 50% of studies from before 2000 [87].
The reasons for variable patient interest in SDM are worth considering. For example, some patients cite authoritarian physician style and a fear of being labeled difficult as factors [88], and others may feel they lack requisite information or skills specific to the encounter [89]. White race, more education [90], and younger age [83] have been found to predict greater patient participation in medical encounters. It is possible to conclude that patient reluctance to participate is in part a dynamic phenomenon, some of whose sources may be expected to diminish with time.
How EPs feel about SDM
In a 2016 survey, SDM was endorsed by a majority of709 EPs across a range of decision categories, including invasive procedures, diagnostic testing (particularly CT scans), medications, and disposition [91]. At least half of respondents considered it appropriate for use “all or most of the time” in a variety of clinical scenarios (antibiotics for severe sepsis was the sole exception), particularly decisions that are either higher risk or associated with controversy or uncertainty. Similarly, EPs surveyed in 2015 considered SDM to be widely applicable, with over half of cases they see involving a choice among reasonable management options [92]. An overwhelming majority of EPs felt SDM could help reduce unnecessary care [9].
Still, EPs estimated that they use SDM only 58% of the times it would be appropriate [92]. Unsurprisingly, EPs reporting less use of SDM in their practice were more likely to cite obstacles. Most obstacles were patient-related and, notably, ran counter to the evidence. For example, the most frequent concern was that patients would prefer to leave decisions in physicians’ hands. About half of EPs also worried that, given a choice, patients would tend to opt for more aggressive care than they need, would find SDM too complicated, and wouldn’t know how to choose among options [92]. As discussed previously, the evidence does not substantiate these concerns. Most EPs were not concerned that SDM would increase length of stay [92].
Challenges for SDM
SDM faces several challenges. Vulnerable populations are one: patients lacking health literacy and in other ways disadvantaged have been found less willing to participate in medical decisions [51]. They ask fewer questions on average, receive less information, and are less satisfied with their encounters with physicians [93]. Nevertheless, evidence suggests they can derive at least as much benefit from SDM as other patients, perhaps more [94]. To be equitable, SDM should be made accessible to all subpopulations able to benefit from it. Determining optimal approaches for patients of wide-ranging knowledge and abilities, however, adds complexity to the task.
A second potential hurdle is that SDM is widely believed to require physician education and training [95,96], if only to encourage its use. Uptake to date has indeed been modest [92], and untrained physicians engage patients to a lesser degree than they believe themselves to [97]. Training can impart more uniform skills and standards, and familiarize physicians with the use of DAs. However, effective patient engagement starts with skills largely already available to (even essential for) physicians: listening, explaining, and expressing compassion.
Probably the greatest difficulties for SDM, however, are the limitations of physicians’ information base and its application to the individual patient—obstacles to effective and evidence-based practice regardless of the level of patient engagement. Physicians have been found to lack salient and up-to-date knowledge [98,99], and may settle for unsupported theory based on physiologic explanations [63]. They are prone to biases [63,100] and uncomfortable with uncertainty [101]. The sheer volume of published research findings leaves physicians largely dependent on secondary information sources; these vary in quality and coverage.
Research findings can offer false hope of benefits in several ways. Reversal of standard-of-care practices is common [102], in part because science is necessarily a dynamic endeavor, but also because of flawed methodologies and because of publication bias favoring positive studies [103]—commercial interests lend sizeable financial incentives for these. Harms have been shown to be much less routinely evaluated and reported than benefits, in the research literature [104]. And studies often fail to indicate how the relative benefits and harms of an intervention may differ across patients, and to provide evidence in the form of pretest probabilities allowing a Bayesian approach to decision making [105].
Clinical guidelines should in theory provide objective, up-to-date evidence summaries to aid decision making, but two problems currently limit their utility. First, many lack impartiality, due to a heavy presence of financial and other conflicts of interest among guideline authors and panel chairs [106]. Second, in aiming to reduce practice variation, guidelines typically default to strong recommendations which de-emphasize patient preference [107,108]. Uniformity of practice is an unreasonable goal when no single approach to management is clearly superior.
Guidelines should offer flexible approaches and encourage patient engagement, describing potential harms as well as benefits for each approach. Otherwise, they pit individual choice against recommendations that may be held up as a standard of practice, creating tension for physicians and patients alike. DAs were created to address this need. They are becoming available online in increasing numbers (Table 2).
A practical guide to SDM
Hoffmann et al [95]. have proposed five questions to guide SDM (Table 3). Note that DAs, where available, can be a helpful adjunct but cannot currently be considered essential; SDM with and without DAs has yet to be formally compared.
Information provided to patients should be presented at a level of detail commensurate with patients’ interest and intellectual ability, and, where knowable, with the potential significance to the patient of the choice to be made. Caverly et al. [109] argue that when patients find information too technical or detailed they may miss the bottom line. They offer these simple talking points for patients weighing the decision to have a CT scan: (1) “the lifetime risk of developing cancer from exposure to radiation from CT scans appears to be real,” (2) “we as clinicians take this risk seriously enough to consider other options for testing that do not use radiation, including physical exam and/or watchful waiting,” and (3) “the small magnitude of the risk is vastly outweighed by the benefits of appropriate imaging.”
What if the patient wants care that will not help? Fenton et al. [110] suggests a 6-step approach (Table 4). Mangione-Smith et al. [111] found that offering parents a contingency plan when children with respiratory symptoms were not given an antibiotic was associated with higher mean satisfaction scores. Importantly, SDM does not obligate physicians to provide the care patients ask for. SDM respects patient autonomy, but autonomy is not the only consideration [112]; refraining from giving non-beneficial care is part of non-maleficence, or doing no harm, and explaining a decision not to provide care has value as patient education [113].
What if instead the patient declines care that the physician feels is important? For example, when the risk of serious disease is low but the stakes are high? Situations will inevitably arise in which a patient’s decision creates discomfort for the physician. With or without SDM, patient autonomy amounts to the freedom to refuse care, assuming a patient has decisional capacity and is sufficiently informed. A systematic review on the question of whether SDM influences medical malpractice litigation found insufficient empirical data to draw conclusions, but some evidence suggested that disregard of patient preferences and failure to provide information increased risk [114]. Interestingly, most EPs already assume that using and documenting SDM lowers medicolegal risk [91].
When a physician is uncomfortable with a patient’s decision, additional safeguards should be utilized where possible, such as ED observation or early follow-up, and the discussion should be documented. SDM should in general be documented, though there is as yet no standard approach for doing so.
CONCLUSION
Many nonpatient factors influence medical decision making. Overwhelmingly, these tend in the direction of encouraging more health care. The result is often medically unnecessary care, or overuse, which not only adds needless cost but exposes patients to avoidable risks. Physician assumptions about patient preferences also affect medical decisions and contribute to unnecessary care. These assumptions are wrong surprisingly often, and even when correct overlook two of patients’ most consistent priorities: communication and empathy by their physicians. By providing balanced information so patients understand their options and any trade-offs involved, encouraging them to voice their preferences and values, and engaging them (to the extent appropriate or desired) in decision making, physicians are in a better position to address patients’ concerns without the use of tests and treatments patients neither need nor value.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
Overuse of health care occurs on a wide scale and presents a threat to patient safety. Overuse of diagnostic testing in particular is a problem in emergency medicine. Fear of missing a diagnosis, fear of law suits, and perceived patient expectations are among the explanations most frequently cited by emergency physicians.
What is new in the current study
Shared decision making–informing and engaging patients, and eliciting their preferences for care–is an underutilized resource for tailoring medical care to the individual patient. Evidence indicates that patients who engage in it often opt for less aggressive approaches to care. Shared decision making has the potential to reduce the delivery of care that may be neither beneficial to, nor desired by, patients.


