AbstractObjectiveWe investigated whether patients with out-of-hospital cardiac arrest (OHCA) due to an acute myocardial infarction without cardiogenic shock required higher doses of vasopressors with low targeted temperature management (TTM) after return of spontaneous circulation.
MethodsWe included consecutive comatose patients resuscitated from OHCA between January 2011 and December 2013. Patients with return of spontaneous circulation, regional wall motion abnormality on echocardiography, and coronary artery stenosis of ≥70% on percutaneous coronary artery angiography were enrolled. These patients received 36°C TTM or 33°C TTM following approval of TTM by patients’ next-of-kin (36°C and 33°C TTM groups, respectively). The cumulative vasopressor index was compared between groups.
ResultsDuring induction phase, dose of vasopressors did not differ between groups. In the maintenance phase, the norepinephrine dose was 0.37±0.57 and 0.26±0.91 µg·kg-1·min-1 in the 33°C and 36°C TTM groups, respectively (P<0.01). During the rewarming phase, the norepinephrine and dopamine doses were 0.49±0.60 and 9.67±9.60 mcg·kg-1·min-1 in the 33°C TTM group and 0.14±0.46 and 3.13±7.19 mcg·kg-1·min-1 in the 36°C TTM group, respectively (P<0.01). The median cumulative vasopressor index was 8 (interquartile range, 3 to 8) and 4 (interquartile range, 0 to 8) in the 33°C and 36°C TTM groups, respectively (P=0.03).
INTRODUCTIONCoronary artery disease is the cause of up to 90% of out-of-hospital cardiac arrest (OHCA) cases, which is a leading cause of death in the developed world [1-3]. Therefore, international guidelines recommend development of protocols to treat acute myocardial infarction (AMI) and establish coronary reperfusion, based on local requirements, in areas with frequent AMIs. International guidelines also recommend the use of targeted temperature management (TTM) for treating OHCA in patients with primary ventricular fibrillation or ventricular tachycardia, and consideration of TTM for other rhythms and for in-hospital cardiac arrests [4]. However, hemodynamic instability is common in comatose OHCA survivors, because of post-cardiac arrest syndrome, and may be exacerbated by induction of TTM [5].
Previous studies have suggested that systemic vascular resistance increases with TTM, while cardiac output decreases in parallel with the heart rate [6]. However, other studies in patients with vasopressor-dependency after cardiac arrest showed that induction of TTM was not associated with a decrease in mean arterial pressure or increase in vasopressor requirement, and TTM was not associated with total cumulative vasopressor index (CVI), survival, or a good outcome [7,8]. However, these studies investigated the relationship between TTM and vasopressor requirements in the management of OHCA with cardiogenic shock, in which vasopressors are needed to increase myocardial contractility. This fact led us to question whether or not OHCA due to AMI without cardiogenic shock requires vasopressors during TTM. Therefore, this study evaluated whether patients with OHCA due to AMI who did not have cardiogenic shock immediately after return of spontaneous circulation (ROSC) required higher doses of vasopressors or inotropes during TTM.
METHODSStudy design and settingThis was a prospective observational study, which included a cohort of consecutive comatose patients (Glasgow Coma Scale ≤8), who were resuscitated from OHCA and admitted to the tertiary care center at a single hospital between January 2011 and December 2013. Our institutional review board approved the study protocol. Written informed consent was obtained from the next-of-kin of all patients.
ParticipantsAdults (≥18 years) who presented to the emergency department of a single tertiary care center with OHCA were considered for enrollment. Patients with ≥70% coronary artery stenosis by angiography were designated as an AMI with a culprit lesion [9]. We enrolled patients with sustained ROSC, regional wall motion abnormality on echocardiography, and ≥70% coronary artery stenosis by angiography. We excluded patients with the following: 1) a diagnosis of stroke, based on brain computed tomography, 2) a comatose state or terminal illness before OHCA, 3) asphyxia as the cause of the arrest, 4) systolic blood pressure <90 mmHg and a cardiac index of <2.6 L·min-1 ·m-2 immediately after ROSC, 5) body temperature ≤32°C immediately after ROSC, 6) normal coronary angiography immediately after ROSC, or 7) lack of consent from the next-of-kin for percutaneous coronary intervention. We used a Vigileo Monitor (Edwards Lifesciences Corporation, Irvine, CA, USA) to measure the cardiac index. We applied 36°C TTM for patients whose next-of-kin did not approve of 33°C TTM (36°C TTM group), or 33°C TTM for patients whose next-of-kin approved of this intervention (33°C TTM group).
Data sources and measurementIn the 33°C TTM group, TTM was induced with intravenous rapid infusion of 20 mL/kg of 4°C saline solution and by surface cooling with the Arctic Sun Temperature Management System (Medivance, Louisville, CO, USA). The target temperature was 33°C; however, temperatures between 32°C and 34°C were permitted. The cooling duration was 12 hours after reaching 33°C. Temperature was monitored in the esophagus and bladder. Subjects were rewarmed at a target rate of 0.25°C/hr. In the 36°C TTM group, surface cooling with the Medi-Therm II thermal regulating system (Gaymar Industries, Orchard Park, NY, USA) as a cooling blanket plus ice packs was used to attain a target temperature of 36°C. Temperature was monitored in the rectum and tympanic membrane. Sedation with propofol or benzodiazepines was recommended to control shivering. Paralysis was intermittently employed during the induction of TTM. Fluid infusion and use of vasopressors and inotropes helped in achieving a urine output of ≥0.5 mL·kg-1 ·hr-1 and a mean arterial pressure of ≥80 mmHg. The choice of specific vasopressors was based on the individual clinician’s preference. The induction phase was defined as the first 8 hours following ROSC in the 36°C TTM group and as the time necessary to attain a body temperature of 33°C following ROSC in the 33°C TTM group. The maintenance phase was defined as the 12 hours following the induction phase in both groups. The rewarming phase was defined as the time interval from when the maintenance phase was terminated until the target temperature of 36°C was reached in both groups. We measured the CVI as the primary outcome during TTM in both groups. The CVI is a previously published scale based on potency and dose of vasopressors, which assigns points for equivalent doses of commonly used vasopressor agents, and quantifies the overall degree of vasopressor support [10,11].
VariablesData on age, sex, location of arrest, call-to-first cardiopulmonary resuscitation (CPR) attempt interval, call-to-ROSC interval, door-to-balloon time, cardiac output immediately after ROSC, in-hospital mortality, and initial cardiac rhythm at the time of OHCA were measured. We recorded vasopressor dose when it was changed, and cerebral performance category (CPC), core temperature, systolic arterial pressure, and diastolic arterial pressure every hour. To calculate the multiple organ dysfunction score (heart rate, central venous pressure, mean arterial pressure, fraction of inspired oxygen, partial pressure of oxygen, serum creatinine level, total bilirubin level, platelet count, and Glasgow Coma Scale), we recorded the data at a single constant time point (the first morning values) as recommended by Marshall et al. [12].
Quantitative variables and statistical methodsContinuous data are presented as mean values and standard deviations or median values and interquartile ranges (IQR), according to the distribution of the data. Categorical data are presented as counts and proportions. All statistical analyses were performed using IBM SPSS Statistics ver. 19.0 (IBM, Armonk, NY, USA). We compared the two groups using an independent t-test, the Mann-Whitney test, and Fisher’s exact test. Values of P<0.05 were considered significant.
RESULTSGeneral characteristicsA total of 82 consecutive comatose patients with acute myocardial infarction as the cause of OHCA admitted to the hospital in the study period were treated with TTM (Fig. 1). The mean age of enrolled patients was 58.18±16.35 years, the call-to-first CPR attempt interval was 13.04±6.39 minutes, the call-to-ROSC interval was 47.87±17.20 minutes, the door-to-balloon time was 36.07±6.92 minutes, and the length of stay was 15.59±14.47 days. The median multiple organ dysfunction score was 8 (IQR, 6 to 10), the median CVI was 4.5 (IQR, 0 to 8), and the mean cardiac output was 47.35±10.93%. The mean time to attain a body temperature of 33°C following ROSC was 5.27±1.82 hours. Other clinical characteristics of patients are summarized in Table 1.
The mean age was 64.29±14.00 years in the 36°C TTM group and 51.61±16.84 years in the 33°C TTM group (P<0.01). The mean call-to-first CPR attempt interval was 12.33±4.51 minutes in the 36°C TTM group and 14.09±8.43 minutes in the 33°C TTM group (P=0.70). The mean call-to-ROSC interval was 45.61±13.44 minutes in the 36°C TTM group and 51.21±21.41 minutes in the 33°C TTM group (P=0.39). The mean door-to-balloon time was 35.98±6.15 minutes in the 36°C TTM group and 36.21±8.03 minutes in the 33°C TTM group (P=0.63). The mean length of stay was 14.45±16.05 days in the 36°C TTM group and 17.27±11.77 days in the 33°C TTM group (P=0.39). The median multiple organ dysfunction score was 8 (IQR, 5.5 to 10.5) in the 36°C TTM group and 8 (IQR, 7 to 10) in the 33°C TTM group (P=0.87). The mean cardiac output was 46.38±10.23% in the 36°C TTM group, and 48.79±11.90% in the 33°C TTM group (P=0.35). We compared CPC between the two groups. Of 49 patients included in the 36°C TTM group, 3 (6.1%) had a CPC of 1, 7 (14.3%) had a CPC of 3, 15 (30.6%) had a CPC 4, and 24 (49.0%) had a CPC 5. Of 33 patients included in the 33°C TTM group, 10 (30.3%) had a CPC 1, 5 (15.2%) had a CPC 2, 9 (27.3%) had a CPC 3, 3 (9.1%) had a CPC 4, and 6 (18.2%) had a CPC 5. CPC scores were better in the 33°C TTM group than in the 36°C TTM group (P<0.01). The comparison of other clinical characteristics between the groups is summarized in Table 2.
Comparison of arterial pressure between the 36°C and 33°C TTM groupsThe minimum arterial pressure was compared between the 36°C and 33°C TTM groups. In the induction phase, this value did not differ significantly between the groups. However, the minimum systolic arterial pressure and mean arterial pressure were lower in the 33°C TTM group than in the 36°C TTM group in the maintenance phase. The minimum systolic arterial pressure, diastolic pressure, and mean arterial pressure were lower in the 33°C TTM group than in the 36°C TTM group in the rewarming phase (Table 3). The arterial pressure and body temperature graphs of both groups are shown in Figs. 2 and 3.
Comparison of vasopressor doses between the 36°C and 33°C TTM groupsThe median CVI was 4 (IQR, 0 to 8) in the 36°C TTM group and 8 (3 to 8) in the 33°C TTM group (P=0.03). In the induction phase, there were no significant differences in the dopamine, norepinephrine, epinephrine, and vasopressin doses between the groups. In the maintenance phase, patients in the 33°C TTM group required higher doses of norepinephrine than did those in the 36°C TTM group. In the rewarming phase, patients in the 33°C TTM group required higher doses of dopamine and norepinephrine than did those in the 36°C TTM group (Table 4).
DISCUSSIONIn this study that excluded cardiogenic shock, the induction phase of TTM was not associated with a decrease in mean arterial pressure or increase in vasopressor requirement. Some studies in patients with vasopressor-dependency after cardiac arrest also showed that induction of TTM was not associated with decreased mean arterial pressure or increased vasopressor requirement, and TTM was not associated with total CVI, survival, or a good outcome [7,8].
However, this study found a lower arterial pressure in the 33°C TTM group during the maintenance and rewarming phases. The CVI was also higher in the 33°C TTM group. These findings suggest that the vasopressor requirement of the 33°C TTM group was higher than that of the 36°C TTM group during the maintenance and rewarming phases. More than 50% of patients develop late hemodynamic instability and vasodilation requiring administration of vasoactive drugs, and 33°C TTM is associated with hemodynamic alterations, with decreased heart rate, elevated lactate levels, and need for increased vasopressor support compared with TTM at 36°C [13-15].
We assume that these differences resulted from peripheral vascular dilation and development of post-cardiac arrest syndrome during the maintenance and rewarming phases of 33°C TTM. Vasoactive agents are often needed to improve myocardial performance, to counteract vasodilation, and to maintain adequate organ perfusion, but may also cause tissue ischemia as an adverse event [16-18].
This study had some limitations. First, the small sample size of the study could result in selection bias. Second, this study was limited to one center, and a larger study could have had different results. Third, our protocol did not mandate invasive hemodynamic monitoring (e.g., placement of a pulmonary artery catheter). Therefore, we did not evaluate the association between TTM and changes in cardiac output directly.
This study of OHCA due to AMI without cardiogenic shock suggests that the vasopressor requirement increases during the maintenance and rewarming phases in patients treated with 33°C TTM compared with 36°C TTM, although 33°C TTM delivery was not associated with an increased vasopressor requirement during induction.
REFERENCES2. Dekker LR, Bezzina CR, Henriques JP, et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case-control study in acute myocardial infarction patients. Circulation 2006; 114:1140-5.
3. Reichenbach DD, Moss NS, Meyer E. Pathology of the heart in sudden cardiac death. Am J Cardiol 1977; 39:865-72.
4. Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: postcardiac arrest care. 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(18 Suppl 3):S768-86.
5. Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008; 79:350-79.
6. Lewis ME, Al-Khalidi AH, Townend JN, Coote J, Bonser RS. The effects of hypothermia on human left ventricular contractile function during cardiac surgery. J Am Coll Cardiol 2002; 39:102-8.
7. Roberts BW, Kilgannon JH, Chansky ME, et al. Therapeutic hypothermia and vasopressor dependency after cardiac arrest. Resuscitation 2013; 84:331-6.
8. Huynh N, Kloke J, Gu C, et al. The effect of hypothermia “dose” on vasopressor requirements and outcome after cardiac arrest. Resuscitation 2013; 84:189-93.
9. Ambrose JA, Loures-Vale A, Javed U, Buhari CF, Aftab W. Angiographic correlates in type 1 and 2 MI by the universal definition. JACC Cardiovasc Imaging 2012; 5:463-4.
10. Dhainaut JF, Antonelli M, Wright P, et al. Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intensive Care Med 2009; 35:1187-95.
11. Trzeciak S, McCoy JV, Phillip Dellinger R, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med 2008; 34:2210-7.
12. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638-52.
13. Bergman R, Braber A, Adriaanse MA, van Vugt R, Tjan DH, van Zanten AR. Haemodynamic consequences of mild therapeutic hypothermia after cardiac arrest. Eur J Anaesthesiol 2010; 27:383-7.
14. Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol 2002; 40:2110-6.
15. Bro-Jeppesen J, Annborn M, Hassager C, et al. Hemodynamics and vasopressor support during targeted temperature management at 33°C Versus 36°C after out-of-hospital cardiac arrest: a post hoc study of the target temperature management trial. Crit Care Med 2015; 43:318-27.
16. Clutter WE, Bier DM, Shah SD, Cryer PE. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest 1980; 66:94-101.
Fig. 1.Flow chart. The flow diagram shows patients admitted to a single hospital after out-of-hospital cardiac arrest (OHCA) from 2011 to 2013. ROSC, return of spontaneous circulation; GCS, Glasgow Coma Scale; PCI, percutaneous coronary intervention; AMI, acute myocardial infarction; TTM, targeted temperature management. 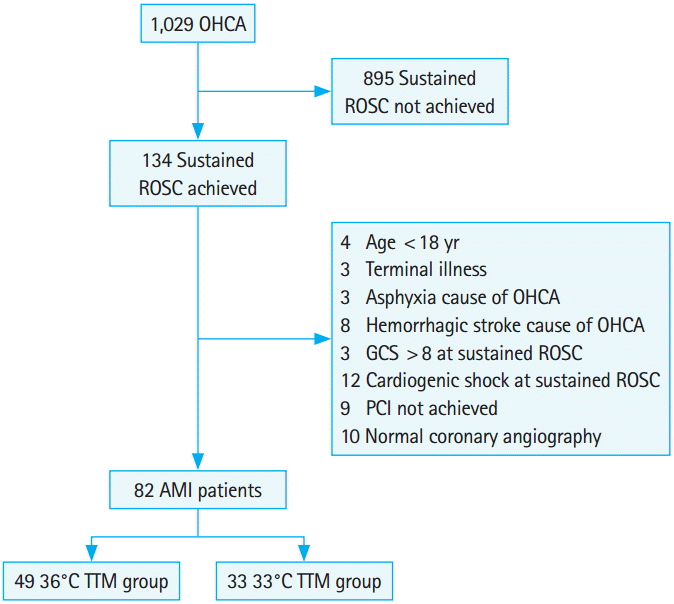
Fig. 2.These images show the arterial pressure in the 36℃ and the 33℃ targeted temperature management (TTM) groups. Dashed lines represent interquartile range while circles represent median value. (A) systolic arterial pressure (SAP), (B) diastolic arterial pressure (DAP), and (C) mean arterial pressure (MAP). There were no significant differences of arterial pressure between groups during the induction phase. However, systolic arterial pressure and mean arterial pressure were lower in the 33℃ TTM group than in the 36°C TTM group during the maintenance phase. Systolic arterial pressure, diastolic pressure and mean arterial pressure were lower in the 33℃ TTM group than in the 36℃ TTM group in rewarming phase. 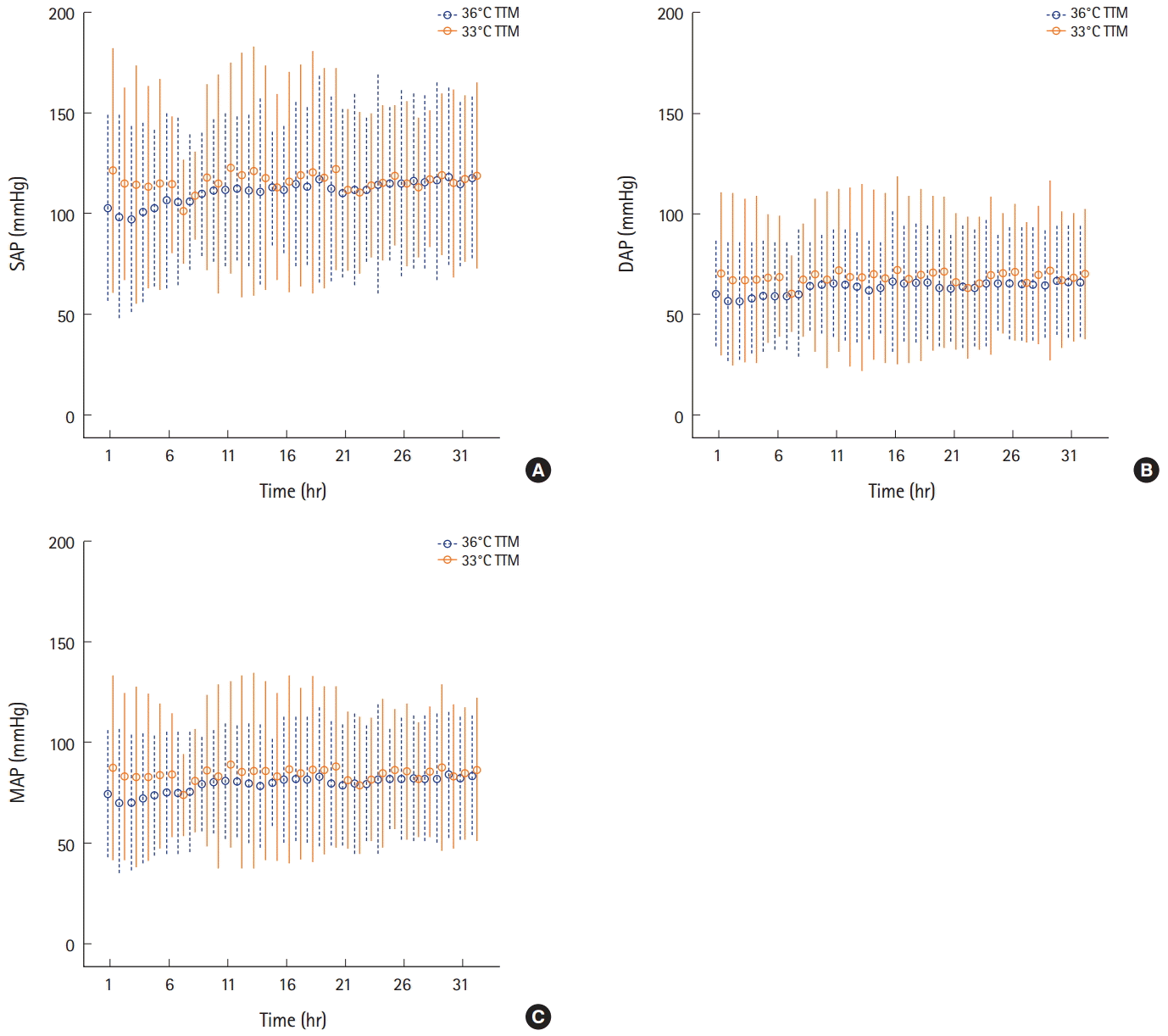
Fig. 3. These images show the mean core body temperature in the 36℃ targeted temperature management (TTM) and the 33℃ TTM groups. Dashed lines represent interquartile range while circles represent median value. 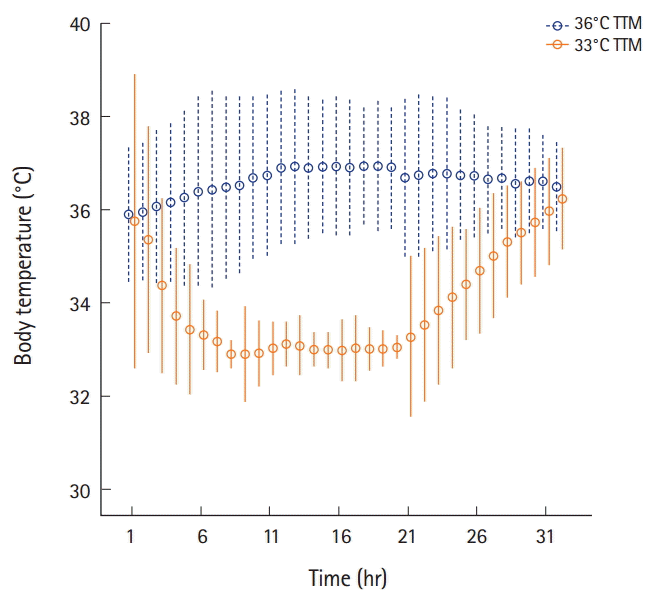
Table 1.General characteristics of study population Table 2.Comparison of general characteristics between the 36°C and 33°C TTM groups Table 3.Comparison of arterial pressures between the 36°C and 33°C TTM groups Table 4.Comparison of vasopressor doses between the 36°C and 33°C TTM groups |
|
|||||||||||||||||||||||||||||||||||||||||