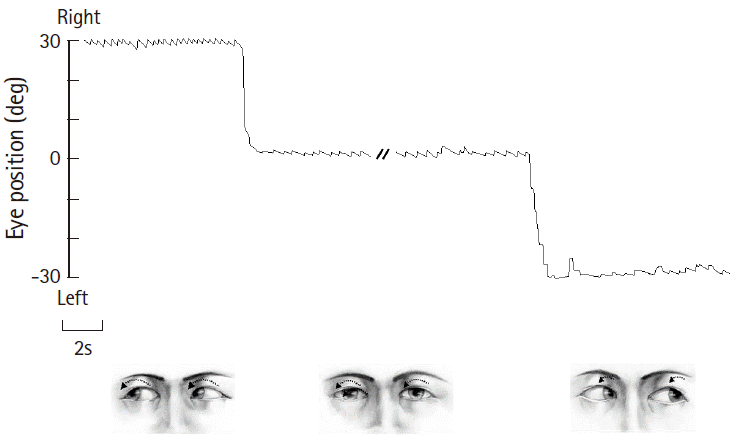INTRODUCTION
Acute dizziness is among the most common causes for visiting the emergency department (ED) [1]. However, the differential diagnosis of dizziness is challenging for several reasons. First of all, dizziness can be a manifestation of conditions varying from life-threatening disorders to normal physiologic responses. Second, no diagnostic confirmatory tool is available for most disorders causing dizziness. Thus, the diagnosis is largely based on a constellation of clinical features obtained with careful history taking and bedside examinations [2]. Neither blood tests nor brain imaging are cost-effective when applied to dizzy patients without discrimination [3]. Third, the term “dizziness” may represent various feelings including vertigo, lightheadedness, pre-syncope, unsteadiness, and just not feeling well [4]. The traditional approach to dizziness starts with defining the type of dizziness based on the belief that each type of dizziness reflects a specific underlying cause: vertigo is vestibular, presyncope is cardiovascular, disequilibrium is neurologic, and nonspecific dizziness is psychiatric or metabolic [4]. However, some patients have difficulty describing their specific type of dizziness [5]. Furthermore, vestibular disorders may present with various patterns of dizziness and cardiac disorders/systemic hypotension can give rise to vertigo along with vestibular signs [6]. Thus, the traditional approach of relying heavily on the “quality of the symptom” faces challenges [3]. A more recently proposed approach to dizziness begins with classifying the type of dizziness/vertigo into acute prolonged spontaneous dizziness/vertigo, recurrent spontaneous dizziness/vertigo, recurrent positional vertigo, or chronic persistent dizziness and imbalance (Table 1). This new approach is considered more practical and more helpful in organizing a differential diagnosis in each category.
In this review, we will focus on the diagnosis and management of acute spontaneous and recurrent positional vertigo, which are the most common types of dizziness/vertigo presenting in the ED.
ACUTE SPONTANEOUS DIZZINESS/VERTIGO
Patients in this category develop acute dizziness/vertigo without triggering factors [2]. The dizziness/vertigo mostly accompanies autonomic symptoms, such as nausea/vomiting and unsteadiness. The attack may be transient (<24 hours) or prolonged. Vestibular neuritis, a benign inflammatory disorder involving the vestibular labyrinth, and stroke are the key disorders for differential diagnosis in this presentation type.
In contrast to the traditional belief that dizziness/vertigo usually accompanies other neurological signs/symptoms in cerebrovascular disorders, isolated vascular vertigo is increasingly identified by virtue of recent developments in clinical neurotology and neuroimaging [7]. Indeed, dizziness/vertigo and imbalance are the most common symptoms in vertebrobasilar ischemia, which comprises up to 20% of all ischemic strokes [8,9]. Recent prospective studies using a large database reported dizziness as a presenting symptom in 47% to 75% of patients with posterior circulation strokes [10,11]. It is important to differentiate isolated vascular vertigo from more benign disorders involving the inner ear since the therapeutic strategy and prognosis differ between these conditions [12]. Misdiagnosis of acute strokes may result in significant morbidity and mortality, while over diagnosis of vascular vertigo can lead to costly work-ups and medication [12]. The introduction of diffusion-weighted magnetic resonance imaging (MRI) has greatly enhanced the infarction detection in patients with vascular dizziness/vertigo, especially due to impaired posterior circulation [13]. However, well-organized bedside neurotologic evaluation is more sensitive than MRI in detecting acute infarction as a cause of spontaneous vertigo of >24 hours, especially during the first 48 hours [14-16].
History taking
Even though neurotological examination plays a vital role in the differential diagnosis of acute spontaneous prolonged dizziness/vertigo, history taking should include preceding infections, vascular risk factors (such as hypertension, diabetes, dyslipidemia, smoking, and cardiac disease), and associated headache or neck pain. Even without other signs suggestive of a central pathology, acute dizziness/vertigo associated with unprecedented severe headache or neck pain strongly indicates a cerebrovascular disorder [17].
Patients visiting the ED with dizziness/vertigo have a two-fold (95% confidence interval [CI], 1.35 to 2.96; P<0.001) higher risk for strokes/cardiovascular events than those without dizziness/vertigo in a 3-year follow-up study [18]. Furthermore, patients with vertigo and three or more vascular risk factors have a 5.51-fold higher risk for strokes (95% CI, 3.10 to 9.79; P<0.001) than those without risk factors [18]. Other studies adopting the ABCD2 score [19], a clinical prediction tool to assess the risk of strokes after a transient ischemic attack, found a cerebrovascular event only in 1.0% of ED patients with dizziness and a score of three or less compared to 8.1% in patients with a score of four or more [20]. Of note, 27% of patients with a score of six or seven suffered from cerebrovascular episodes [20]. Thus, the ABCD2 score may predict cerebrovascular attacks in patients with transient vertigo.
Evaluation
Nystagmus
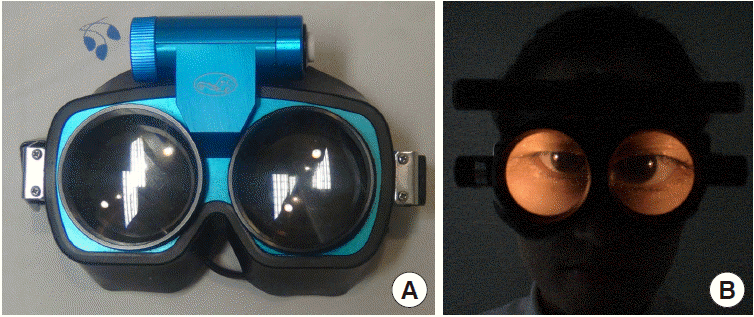
Patterns of spontaneous nystagmus, when present, are most informative in evaluating patients with acute dizziness/vertigo [21]. For proper evaluation of spontaneous nystagmus, one should observe the direction and the effect of gaze on the intensity and direction of the nystagmus. In vestibular neuritis, spontaneous nystagmus is torsional-horizontal beating away from the lesion side (Fig. 1) [22]. The nystagmus typically increases during gaze in the direction of the spontaneous nystagmus and decreases during gaze in the opposite direction (Alexander’s law, Fig. 1) [23]. Since peripheral vestibular nystagmus is markedly suppressed by visual fixation, proper observation of nystagmus requires the removal of visual fixation using Frenzel glasses (Fig. 2) [24]. In contrast, the direction and fixation effect may be variable in central vestibular nystagmus. Accordingly, when the characteristics of nystagmus do not conform to those of peripheral vestibular nystagmus, it should be considered central [25-28]. However, even unidirectional horizontal-torsional nystagmus suppressed by visual fixation should not be simply regarded as peripheral unless other findings, such as positive head impulse test (HIT) or caloric paresis, are supportive of peripheral vestibular lesions. The distinguishing features of peripheral and central nystagmus are summarized in Table 2.
Various bedside maneuvers can induce nystagmus or modulate pre-existing spontaneous nystagmus. Even in patients with spontaneous nystagmus, pattern modulation may reveal the underlying pathology or aid in diagnosis. In patients with compensated vestibulopathy, induction of nystagmus by various maneuvers is crucial in revealing the underlying vestibular imbalance [29,30]. Gaze-evoked nystagmus (GEN) refers to nystagmus that develops when patients take eccentric eye positions. As GEN is caused by impaired gaze-holding in those positions, which causes centripetal drift of the eyes, GEN beats in the direction of gaze [31,32]. GEN is one of the most sensitive ocular motor signs for central pathologies in the patients with acute vestibular syndrome [13,14]. Headshaking nystagmus (HSN) can be induced using either passive (by examiner) or active (by patients) head oscillation. The patient’s head is pitched forward by about 30° to bring the horizontal semicircular canals into the plane of stimulation. The head is then shaken horizontally in a sinusoidal fashion at a rate of about 2 to 3 Hz with an amplitude of 20° for 15 seconds [33]. In unilateral peripheral vestibulopathy, HSN initially beats to the intact side, decays over 20 seconds, and then goes through a weak reversal [34]. In contrast, HSN patterns may vary in central vestibular disorders. In general, central patterns of HSN include unusually strong HSN elicited by weak head-shaking, intense HSN in patients without caloric paresis, ipsilesional HSN, HSN in the opposite direction of spontaneous nystagmus, and perverted HSN (i.e., vertical or torsional nystagmus developing in response to horizontal head-shaking) [33,35-37].
HIT
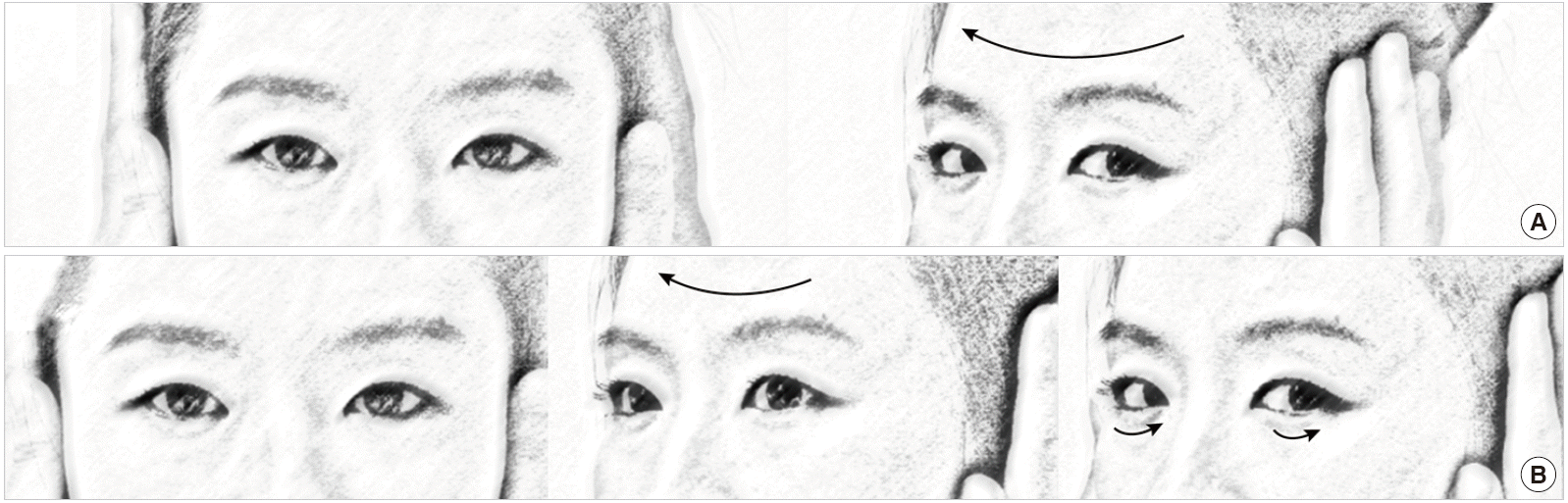
HIT is the most effective method for detecting loss of vestibular function at the bedside [38]. For HIT, the examiner asks the patient to fixate on a target in front of the eyes and briskly turns the patient’s head horizontally. The head impulse should be unpredictable with a low amplitude (10° to 20°) and high acceleration (2,000°/s2 to 4,000°/s2) of head rotation [39]. If the vestibulo-ocular reflex is working normally, HIT will generate a compensatory eye movement in the opposite direction of the head rotation with equal amplitude, holding the gaze steady. In contrast, HIT toward the side of a peripheral vestibular lesion will give rise to a re-fixation catch-up saccade at the end of head motion to bring the image of the target back to the fovea. This corrective saccade (overt saccade) indicates a decreased vestibulo-ocular reflex in patients with peripheral vestibular deficits (Fig. 3). While an overt saccade is observed in most patients with acute peripheral vestibular disorders, HIT is usually normal in central vestibular lesions [40]. Thus, one should suspect a central pathology if a patient with acute vertigo and spontaneous nystagmus exhibits normal HIT [13]. A refixation saccade in a different plane (i.e., vertical catch-up saccade after horizontal rotation) also suggests a central lesion [41-43]. However, bedside HIT may be negative when the vestibular deficits are partial [44] or the covert saccades complement the vestibular deficits [39].
Ocular misalignment
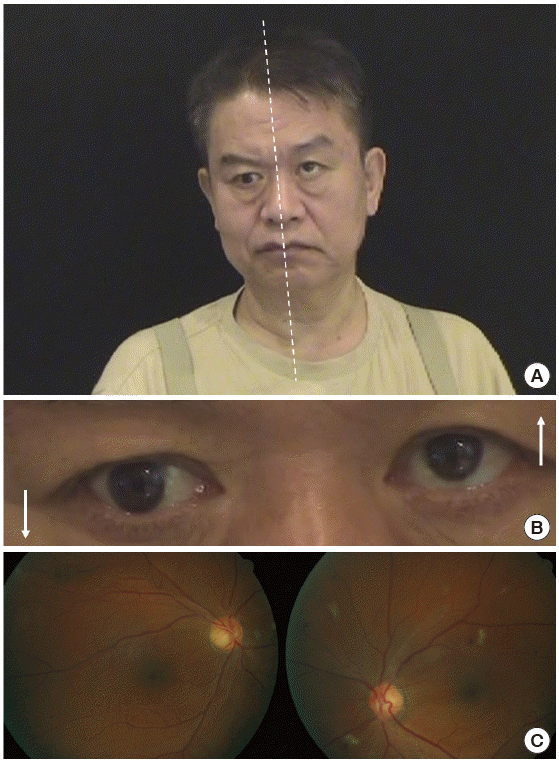
Ocular misalignment occurs frequently in central vestibulopathies, and should be determined in nine cardinal gaze positions along with limitations in the range of eye movements. Skew deviation refers to vertical ocular misalignment resulting from vestibular tone imbalance [45]. The presence of skew deviation may be inferred by vertical diplopia and can be confirmed with the cover test. With one eye covered, a corrective vertical movement of the opposite eye to fixate on a target indicates the presence of skew deviation. Skew deviation may occur in any acute lesion involving the posterior fossa, but the majority of cases are seen in association with brainstem stroke [46]. Skew deviation is typically observed as a component of the ocular tilt reaction (OTR) that includes head tilt, ocular torsion, and skew deviation (Fig. 4). As a rule, the head is tilted toward the side of the lower eye and the ocular torsion occurs in the same direction with the upper poles of the eyes rotating toward the lower eye [47]. Lesions below the lower pons cause ipsiversive OTR, while more rostral lesions induce contraversive OTR.
Balance
The severity of imbalance and falling direction may provide important clues for underlying vestibular impairments. In general, patients with unilateral peripheral vestibular loss, such as vestibular neuritis, can maintain balance even when the dizziness/vertigo is very severe [17]. In contrast, severe imbalance that impairs sitting or standing usually indicates a central pathology [17].
Brain imaging
The use of neuroimaging studies is increasing for ED patients with dizziness. Nevertheless, the sensitivity of computed tomography scans to identify strokes in the acute setting is disappointing (26%) [48]. Thus, a negative computed tomography does not exclude the probability of stroke. MRI is much more sensitive, but the sensitivity is lowest within 24 hours of onset, especially in lesions involving the brainstem or cerebellum [40,48,49]. Thus, serial evaluation is required to confirm a stroke because initial diffusion-weighted MRI may be false negative in 12% to 20% of stroke patients within the first 48 hours [14,50]. Additionally, MRI cannot detect isolated labyrinthine infarctions that may progress to involve portions of the brainstem and cerebellum supplied by the anterior inferior cerebellar artery (AICA) [51]. Perfusion imaging may help determine the presence and extent of hypoperfusion, especially when routine MRI, including diffusion-weighted images, are normal [52]. However, the exact role of perfusion imaging needs to be validated in isolated vertigo of vascular origin.
Optimal testing
Recent studies have shown well-organized bedside examination is superior to brain imaging in detecting strokes in a subgroup of patients with acute spontaneous dizziness/vertigo. This includes HINTS, which stands for Head-Impulse-Nystagmus-Test-of-Skew [14]. In a previous study, the presence of negative HIT, direction-changing nystagmus, or skew deviation predicted stroke with 100% sensitivity and 96% specificity in patients with acute vertigo and at least one vascular risk factor after excluding those with a history of recurrent vertigo. In contrast, initial diffusion-weighted MRI was false negative in 12% of these patients, especially within 48 hours of symptom onset [29]. However, HINTS may not be sufficiently robust to detect infarctions involving the AICA territory since HIT is mostly positive due to concurrent labyrinthine infarction [15,53,54]. Indeed, HINTS failed to detect central lesions in five of 18 patients with AICA infarctions [53]. Therefore, detection of central lesions may require additional testing, such as horizontal head shaking [53] or hearing evaluation (HINTS plus) [15,16].
Transient vascular vertigo and isolated labyrinthine infarction
Despite being a common finding in vertebrobasilar ischemia, diagnosis of isolated transient dizziness/vertigo of a vascular origin remains a challenge [9,55,56]. Transient isolated vascular vertigo typically occurs abruptly and usually lasts several minutes [57]. According to a report, 62% of patients with vertigo due to vertebrobasilar ischemia had a history of at least one isolated episode of vertigo, and 19% developed vertigo as the initial symptom [55]. Patients with AICA territory infarctions may have isolated recurrent vertigo, fluctuating hearing loss, and/or tinnitus (similar to Meniere’s disease) as initial symptoms 1 to 10 days prior to permanent infarction [58].
This diagnostic difficulty also applies to isolated labyrinthine infarctions since no confirmatory tool other than a pathologic study is currently available for this condition [59]. As the internal auditory artery, usually an AICA branch, supplies the inner ear, vertebrobasilar ischemic strokes may present with vertigo and hearing loss due to labyrinthine infarction. As isolated labyrinthine damage may precede ponto-cerebellar involvement from an AICA infarction, audiovestibular loss may serve as an indicator of progression into more widespread infarction involving the posterior circulation, mainly in the AICA territory [51,60]. Labyrinthine infarction should be considered in older patients with acute vertigo and unilateral hearing loss, particularly when there is a history of strokes or known vascular risk factors. Because labyrinthine infarctions are not well visualized with current imaging techniques [59], clinicians should consider all the clinical features and laboratory findings available when attempting to determine the cause of acute vertigo and hearing loss [61].
Management
Patients with suspected episodes of vascular vertigo should have a prompt assessment of their cerebral vasculature [12,62]. As non-lacunar mechanisms are more common (47%) than previously thought in acute vestibular syndrome from small infarctions, more aggressive therapies may be indicated to prevent stroke recurrences in vascular vertigo [16,63].
The treatment of vestibular neuritis generally includes supportive care during the acute phase, steroids, and vestibular rehabilitation [64]. Symptomatic care with vestibular suppressants should be used only during the first several days when patients suffer from severe nausea/vomiting and vertigo, as such medications may delay central compensation [23]. The efficacy of corticosteroids is controversial. A recent Cochrane review concluded there is currently insufficient evidence to support the use of corticosteroids in patients with idiopathic acute vestibular dysfunction [65]. Administration of valcyclovir alone, or in combination with glucocorticoids, showed no effect [66]. In contrast, vestibular rehabilitation hastens recovery [67].
RECURRENT POSITIONAL VERTIGO
According to the Barany Society classification of vestibular symptoms [68], positional vertigo is defined as vertigo triggered by and occurring after a change of head position in space relative to gravity. Thus, positional vertigo should be differentiated from headmotion vertigo, which occurs only during head motion, or orthostatic vertigo, which is triggered by and occurs upon rising [68]. Isolated positional vertigo is usually caused by benign paroxysmal positional vertigo (BPPV) [69]. BPPV is characterized by brief spinning sensations, usually lasting less than 1 minute [69]. Vertigo typically develops when a patient gets in or out of bed, rolls over in bed, tilts the head back, or bends forward [69]. BPPV is explained by otoconia dislodged from the macula of the utricular otolith entering the semicircular canals [70]. When there is a change in the position of the head with respect to gravity, otolithic debris moves to a new position within the semicircular canals, leading to a false sense of rotation [70]. BPPV usually arises from the posterior semicircular canal (PC-BPPV), which accounts for 60% to 90% of all cases [69]. However, the proportion of patients with BPPV involving the horizontal semicircular canal (HC-BPPV) may have been underestimated since HC-BPPV is more likely to remit spontaneously than PC-BPPV [71]. BPPV rarely involves the anterior semicircular canal, probably because of its uppermost position where otolithic debris is unlikely to become trapped [72].
PC-BPPV
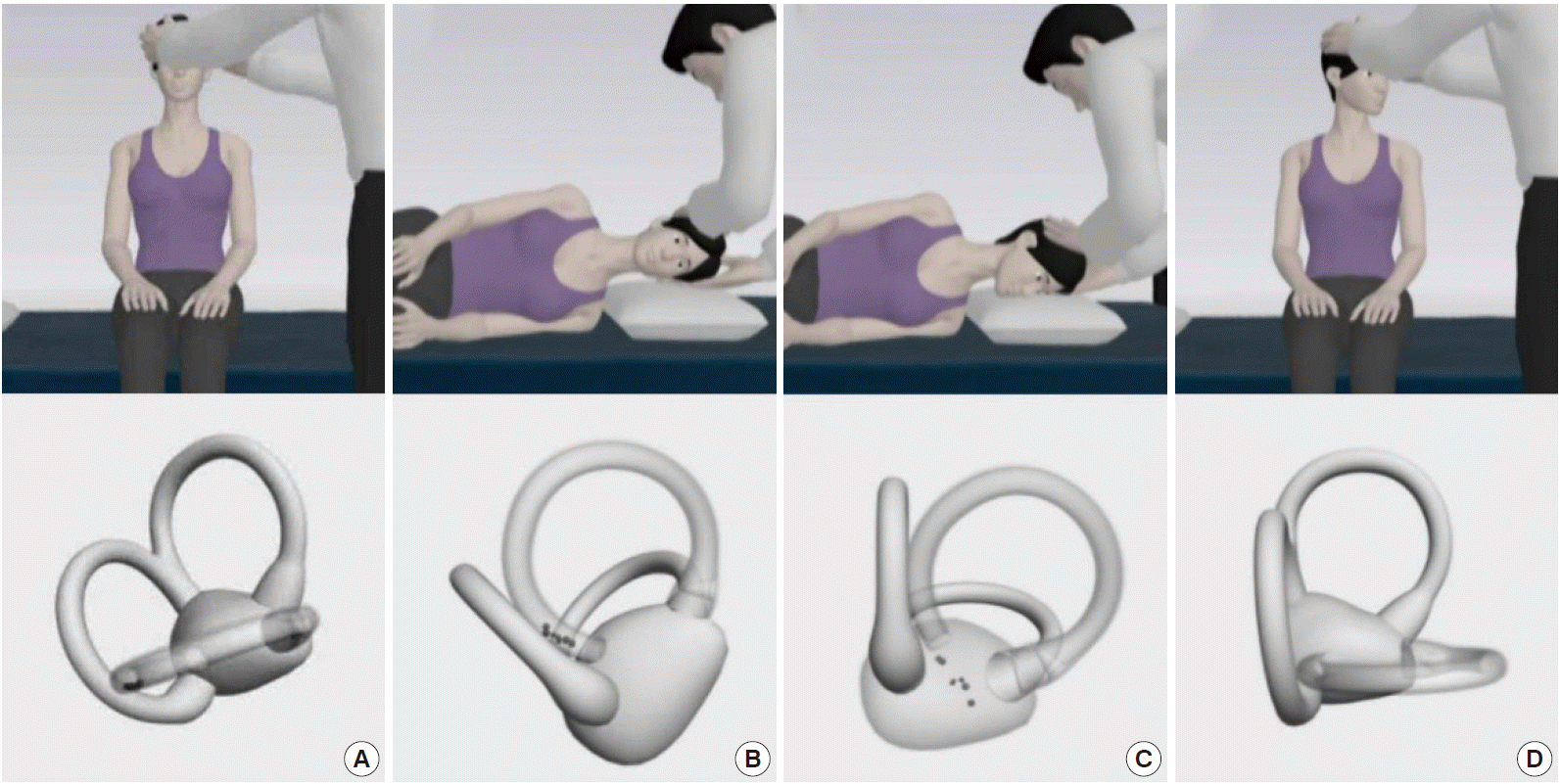
In PC-BPPV, paroxysmal vertigo and nystagmus are typically induced by the Dix-Hallpike maneuver (Fig. 5). During this maneuver, otolithic debris in the ipsilateral posterior canal move away from the cupula (canalolithiasis), and the induced ampullofugal (away from the cupula) endolymph flow stimulates the posterior canal. From this stimulation, upbeating and ipsiversive torsional nystagmus (top poles of the eyes beating toward the downward ear) is evoked. This nystagmus usually occurs after a brief latency (2 to 5 seconds) and resolves within 1 minute [69]. When the patient is moved from the recumbent to sitting position, the direction of nystagmus is reversed (i.e., downbeat and contraversive torsional). This nystagmus decreases with repeated maneuvers (fatigue phenomenon) [73].
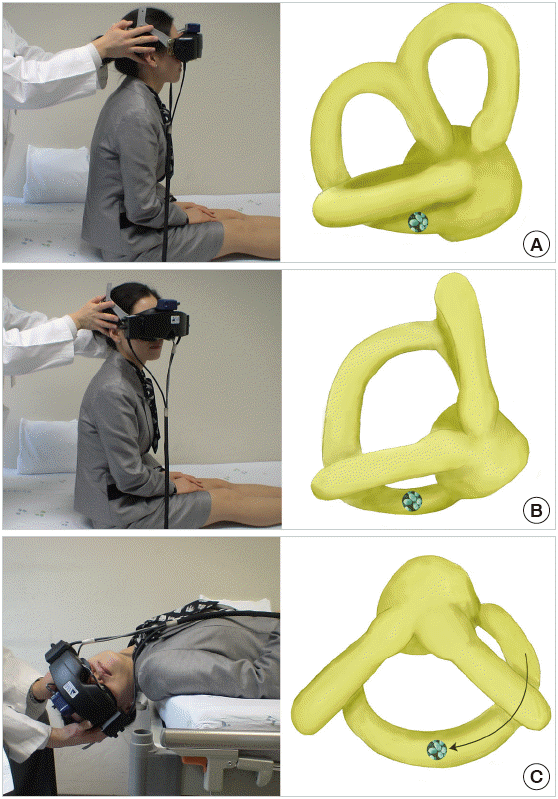
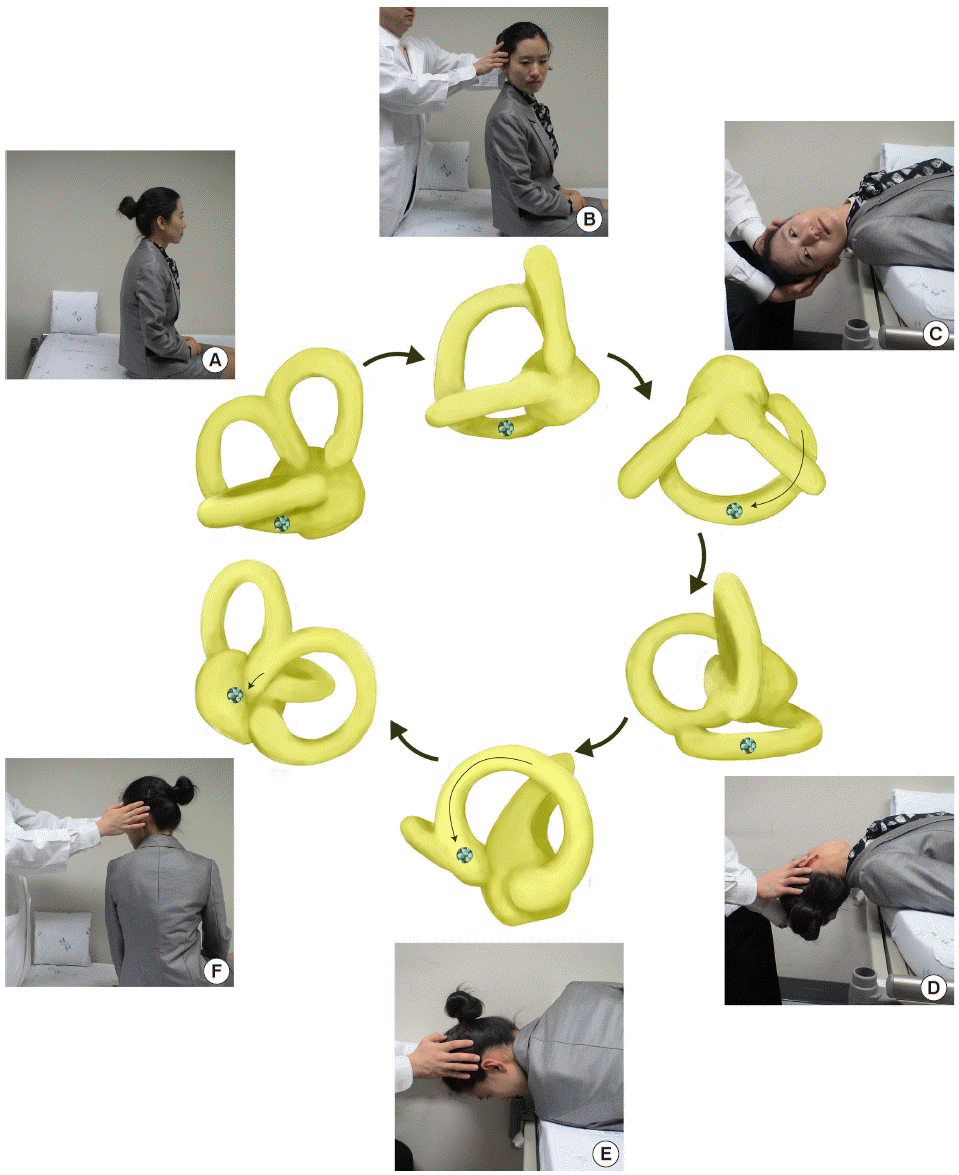
The Epley maneuver, developed to treat PC-BPPV, consists of stepwise 90° rotations of the head toward the unaffected side [77]. The Epley maneuver is performed in five steps, including two from the Dix-Hallpike maneuver. After the Dix-Hallpike maneuver, the head is turned 90° towards the healthy side. The induced nystagmus, if present during this step, would be in the same direction as the one evoked during the Dix-Hallpike maneuver. The head is then turned another 90°, to a facedown position, and the trunk is turned 90° in the same direction, with the patient lying on the unaffected side. The patient is then moved to the sitting position. Otoconia move around the canal with each step of this maneuver and eventually drop out into the vestibule. Each position should be maintained until the induced nystagmus or vertigo dissipates, but always for at least 30 seconds (Fig. 6).
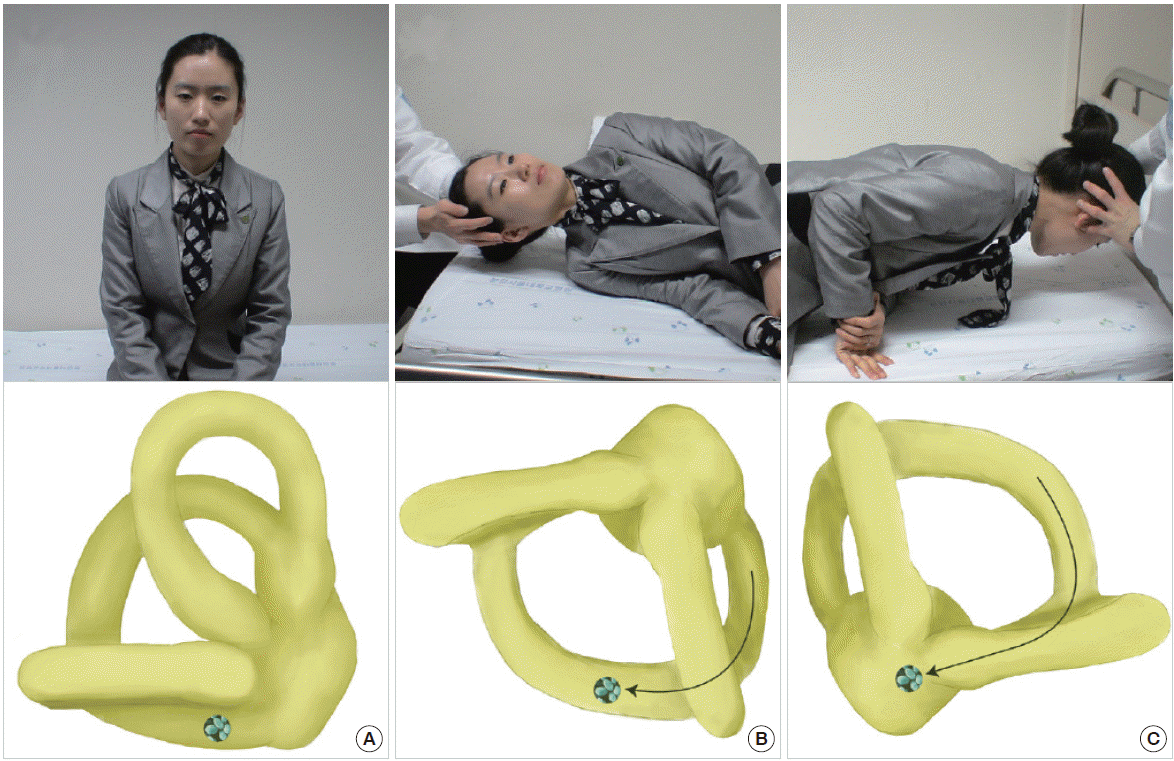
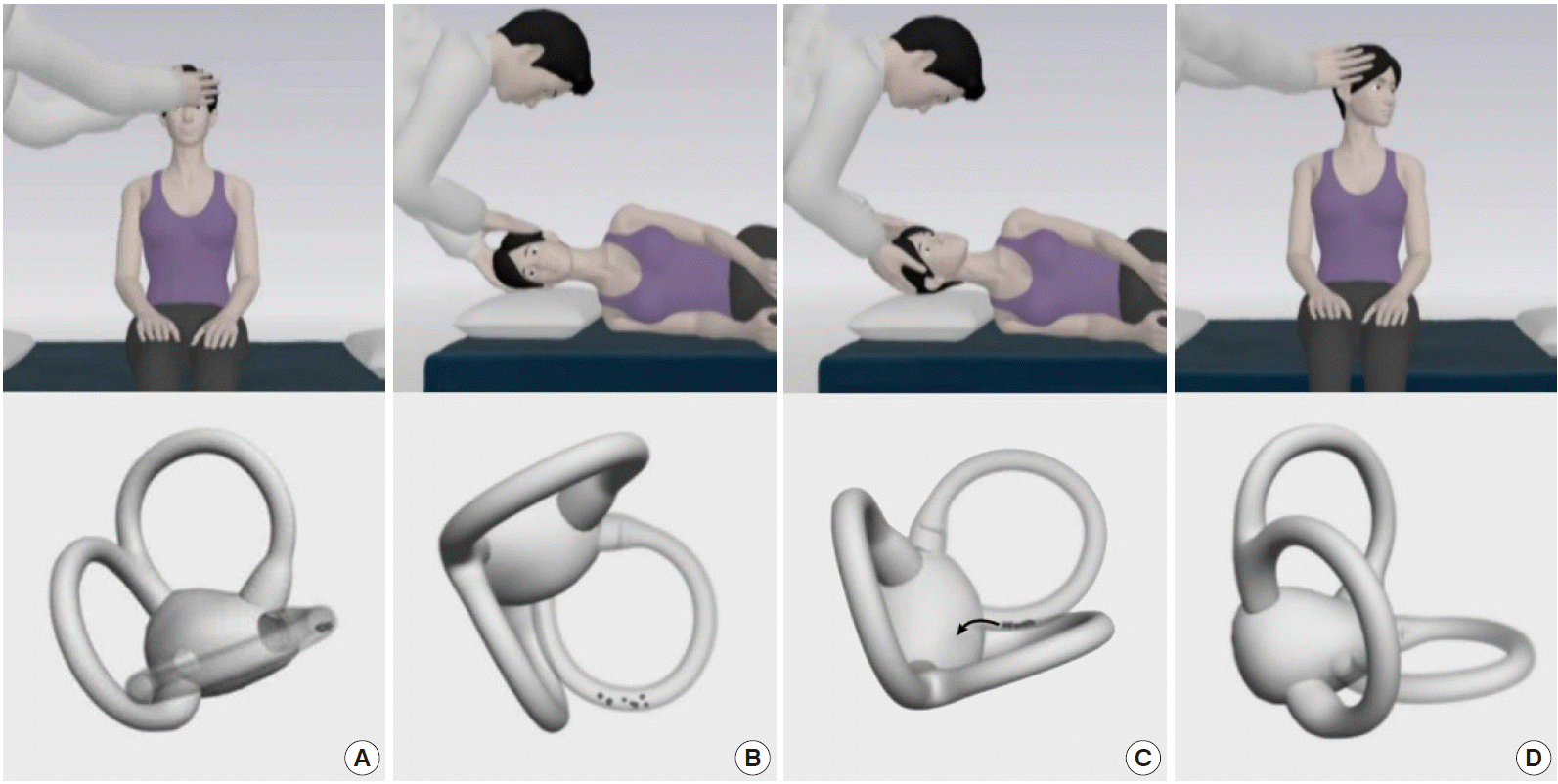
The Semont maneuver is an alternative to the Epley maneuver for treating PC-BPPV, especially for patients with back pain [78]. During this maneuver, the patient makes a quick sweeping movement from the affected to the unaffected side (Fig. 7). Since this movement should be done rapidly (ideally within 1.3 seconds [79]), applying the Semont maneuver can be difficult for older or obese patients.
HC-BPPV
HC-BPPV is characterized by paroxysmal vertigo and direction-changing nystagmus beating towards the lowermost (geotropic) or uppermost (apogeotropic) ear during head turning to either side while supine (head-rolling test) [80]. The geotropic type of HC-BPPV has been ascribed to free-floating otolithic debris in the horizontal canal (canalolithiasis), and the apogeotropic type has been attributed to debris attached to the cupula (cupulopithiasis) or in the anterior arm of the canal [77].
For HC-BPPV diagnosis, the patient is quickly taken from the sitting to supine position with the head lifted 30° on a pillow, to bring the HC into the vertical plane. In this position, lying down nystagmus is observed. Thereafter, a quick 90° lateral head rotation to one side is delivered while the patient remains supine. Then the head is turned to the other side, keeping the head flexed at 30°. The geotropic type of HC-BPPV is diagnosed by horizontal direction-changing nystagmus beating toward the lowermost ear on head turning to either side. Geotropic nystagmus usually has a latency of several seconds, rapidly builds up, and usually dissipates within 1 minute. In contrast, apogeotropic nystagmus beats toward the uppermost ear with a maximum intensity immediately after positioning, and may last more than 1 minute [81]. In HC-BPPV, the affected ear is usually identified by comparing the intensity of nystagmus in each direction [82]. The assumption is that nystagmus is more intense when the head is rotated toward the affected side in the geotropic type, whereas the reverse is true for the apogeotropic type. When the induced nystagmus is symmetric, the direction of lying-down nystagmus may help lateralization. In the geotropic type, lying-down nystagmus usually beats away from the affected side, while beating towards the affected ear is seen in the apogeotropic type [82].
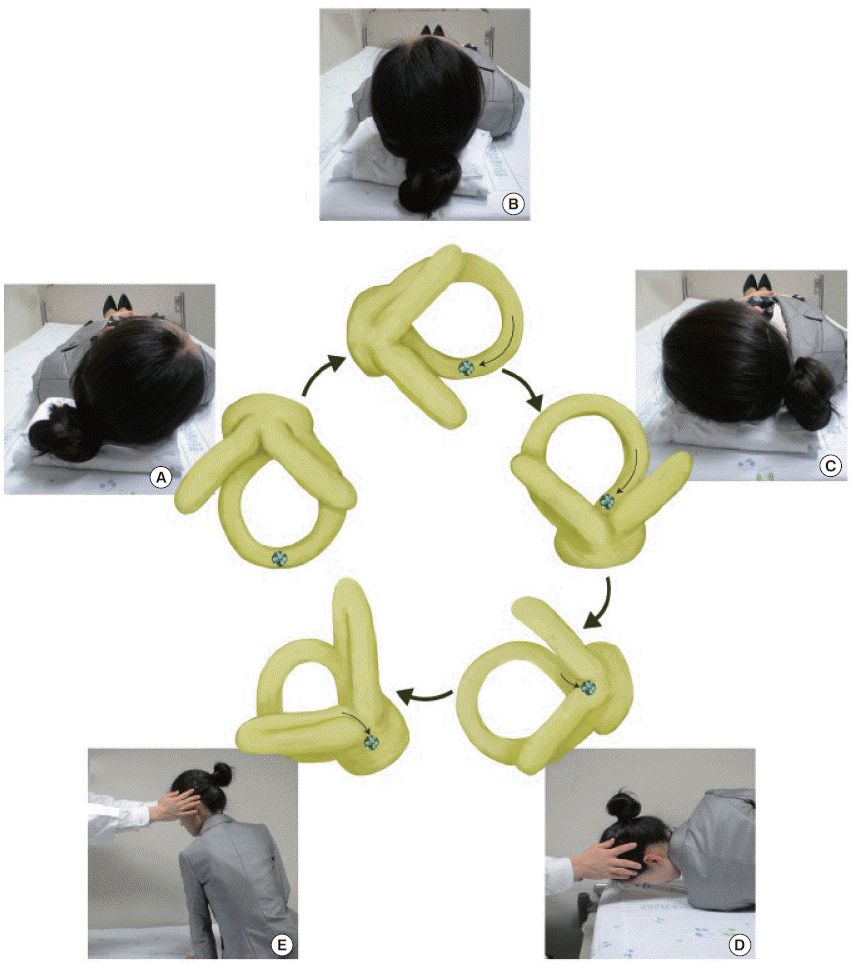
Geotropic HC-BPPV is usually treated with barbecue (360° roll) rotation or the Gufoni maneuver. For barbecue rotation, the patient’s head is rotated 270° with sequential 90° turns from the affected ear down position to the prone position (Fig. 8) [83]. The Gufoni maneuver for geotropic HC-BPPV involves side-lying from a sitting position with the unaffected ear down, head turning toward the floor, and resuming the sitting position (Fig. 9) [84]. Each position should be maintained for 1 to 2 minutes. A recent randomized trial showed that both the barbeque rotation and Gufoni maneuver are more effective than a sham maneuver (success rates of 68%, 61%, and 35%, respectively) [85]. Treatment of apogeotropic HC-BPPV involves maneuvers designed to detach the otolithic debris from the cupula, or to move the debris from the anterior arm of the HC to the posterior arm [86]. Horizontal head shaking for 15 seconds and the Gufoni maneuver are usually adopted for this purpose [87]. During the Gufoni maneuver, designed to treat apogeotropic HC-BPPV, the patient sits upright, looking straight head, and then quickly lies down on the affected side. They remain in this position for 1 to 2 minutes after the nystagmus has stopped or markedly reduces. The head is then quickly turned 45° toward the ceiling and held in this position for 2 minutes, after which the patient slowly resumes the sitting position (Fig. 10). A randomized trial showed significantly higher rates of immediate symptom resolution with the head shaking and Gufoni maneuvers compared to a sham maneuver (62%, 73%, and 35%, respectively) [87].
CONCLUSION AND RECOMMENDATIONS
The evaluation and proper management of acute dizziness/vertigo are very important in the ED. Particular attention should be paid to potentially life-threatening disorders as a cause of acute dizziness/vertigo. A recently proposed approach to dizziness/vertigo begins with classifying the type of dizziness/vertigo presentation. Physicians in the ED should be familiar with examinations to detect central vestibular signs, such as negative HIT, direction-changing nystagmus, and skew deviation, in patients with acute prolonged spontaneous dizziness/vertigo. These signs are more sensitive than brain imaging in detecting stroke presenting with acute isolated dizziness/vertigo. Isolated positional vertigo is almost always caused by BPPV, which can be readily treated with canalith repositioning maneuvers once the involved semicircular canal is determined. Over the past decades, we have achieved marked progress in the evaluation and management of acute dizziness/vertigo. Even with the splendid developments in imaging technology, the diagnosis of acute dizziness/vertigo largely remains the realm of bedside examination.