AbstractObjectiveWe aimed to investigate the causes and clinical and laboratory features of patients with ureteritis observed on intravenous contrast-enhanced abdominopelvic computed tomography (APCT) conducted in the emergency department (ED).
MethodsAll APCTs conducted in the ED from November 2017 to November 2020 were investigated for the presence of ureteritis. The incidence of ureteritis, presumed cause of ureteritis, and clinical as well as laboratory features of patients with ureteritis were retrospectively analyzed.
ResultsUreteritis was observed in 422 out of 7,386 patients (5.7%) who underwent APCTs. The two main reasons for undergoing APCT in the ED were abdominal pain (49%) and infection focus workup (33%). The first major cause of ureteritis was urinary tract infection (UTI) (351 of 422, 83%). Most patients (85%) were febrile, but 208 (59%) exhibited no urinary symptoms such as dysuria, increased frequency, or residual urine sense. The second major cause of ureteritis was ureteral stones (42 of 422, 10%). Thirty-two of 42 patients (76%) had simple obstructive uropathy, while 24% of patients had a combined infection along with an obstruction. Other rare causes were malignancy and the spread of adjacent inflammation.
ConclusionUreteritis was a common finding observed in 5.7% of patients who underwent APCTs at the ED, and most of them were secondary to UTIs and ureteral stones. UTIs can cause ureteritis even without typical symptoms or signs suggestive of UTI, and diagnosis without an APCT can be difficult. More liberal use of APCTs should be considered when the cause of fever is difficult to diagnose.
INTRODUCTIONThe ureter is a tubular structure approximately 20 to 30 cm in length, connecting the kidneys to the bladder. The ureter is histologically composed of three layers (the mucosa consists of transitional epithelium, submucosal connective tissue, and lamina propria), but the histological layered structure cannot be distinguished on computed tomography (CT). Generally, the wall thickness of the ureter in an average adult does not exceed 1 mm, and there is no contrast-enhancement [1]. However, in the presence of ureteritis, the ureter wall thickens to 1 mm or more, and contrast of the ureter wall is enhanced if an intravenous agent is used.
Intravenous contrast-enhanced abdominopelvic CT (APCT) is an essential imaging test frequently conducted in the emergency department (ED) for various reasons, including abdominal pain, fever, and trauma. However, the authors experienced a relatively low interest in the ureter compared to the major abdominal organs. For this reason, we inferred that there might be cases where ureteritis on APCTs was overlooked.
The known causes of ureter abnormalities observed on CT are ureteral malignant neoplasms [2,3], metastasis of other malignant neoplasms [2-4], fibroepithelial polyps [5], ureteritis Cystica [6], tuberculosis [7], amyloidosis [8], inflammation due to urinary tract infections (UTIs) [1,9], non-infectious inflammation caused by indwelling ureteral stents or radiation [1,10], the spread of peripheral inflammation such as pancreatitis or enteritis [1], urinary tract obstruction due to ureteral stones or neoplasia [1], and retroperitoneal fibrosis [9]. However, little is known about the clinical characteristics of patients with ureteritis observed in APCTs performed in the ED. Therefore, this study investigated the causes of ureteritis observed in APCTs conducted in the emergency room and examined the clinical and laboratory (blood and urine tests) characteristics of ureteritis patients.
METHODSEthics statementAfter obtaining approval from the Institutional Review Board of Kangbuk Samsung Hospital (No. 2021-05-032), a retrospective cross-sectional study was conducted. The Institutional Review Board exempted written informed consent due to the retrospective nature of the study. To ensure anonymity, personal information such as patient name, date of birth, and social indentification number were deleted after assigning research subject numbers. This study was conducted in compliance with the World Medical Association Declaration of Helsinki [11].
Study subjectsWe investigated the results of every APCT conducted in the ED of our hospital for 3 years from November 2017 to November 2020. In repeated APCT scans due to revisits of the same patient, only the first APCT was included for analysis. Results of scans of the same patient performed at more than one time during the study period were excluded. Furthermore, patients with missing blood or urine tests or missing clinical data were excluded. Lastly, patients were analyzed retrospectively for the presence or absence of ureteritis on APCTs, and patients with ureteritis were selected as the final study subjects (Fig. 1). The presence or absence of ureteritis was judged based on the radiologistŌĆÖs formal report.
Outcome measuresThe cause of ureteritis and the clinical and laboratory characteristics of patients with ureteritis were analyzed. Ureteritis was defined as a diffuse circumferential urothelial wall thickening greater than 1 mm and contrast-enhancement (┬▒periureteral fat stranding) on APCTs. The cause of ureteritis was presumed to be a UTI if the patient exhibited a fever (or history of fever)+serum infection marker (C-reactive protein [CRP] or procalcitonin [PCT]) elevation +no cause of infection other than acute pyelonephritis (APN) or cystitis (Fig. 2). Ureteral stones were presumed to be the cause of ureteritis if diffuse, circumferential urothelial wall thickening of more than 1 mm and contrast-enhancement was observed in the ureter proximal to the ureteral stone (Fig. 3). Ureteritis caused by ureteral stones was considered to be due to simple obstructive uropathy if there was no pyuria and serum infection marker elevation. On the other hand, if there was pyuria or a positive urine culture and serum infection marker elevation, ureteritis was considered due to a combined infection rather than a simple obstructive uropathy. Additionally, the characteristics of patients with only ureteritis on APCTs (no APN nor cystitis on APCTs) were also analyzed.
Statistical analysisA descriptive analysis was conducted to show the clinical and laboratory characteristics of ureteritis. Graphical methods and Shapiro-Wilk tests were conducted to determine the normality of the continuous variables, but most of the continuous variables did not satisfy normality. Therefore, the Mann-Whitney U-test was used to compare continuous variables. Categorical variables were compared using chi-square or Fisher exact test according to the expected frequency. Continuous variables were expressed as the median (interquartile range), while nominal variables were expressed as frequency (%), and statistical significance was considered with P-values less than 0.05. The statistical analyses were conducted using Stata ver. 15.0 (StataCorp, College Station, TX, USA).
RESULTSWe investigated the results of 10,177 cases of APCTs conducted in the ED of our hospital over 3 years from November 2017 to November 2020. The results of 623 APCT scans on the same patients, performed more than once during the study period, were excluded. Furthermore, 2,103 patients with missing blood or urine tests and 65 patients with missing clinical data were excluded. A final total of 7,386 patients were retrospectively analyzed for the presence or absence of ureteritis on APCTs, and 422 patients (5.7%) with ureteritis were selected as the final study subjects (Fig. 1).
The main reason for undergoing APCT in the ED was differential diagnosis of abdominal pain, followed by fever workup (fever source investigation and differential diagnosis of infectious disease) and trauma (Table 1). Particularly, of the 2,425 patients who underwent APCT for fever workups, ureteritis was observed in 321 (13%) (Table 1).
Among the 422 patients who had ureteritis on APCT during this study period, the cause of ureteritis in 351 cases was UTIs, accounting for 83% of the cases. Additionally, of the 351 patients with ureteritis due to UTIs, 329 (93.7%) had ureteritis and APN or cystitis simultaneously, while 22 patients (6.3%) had only ureteritis without APN or cystitis. The cause of sole ureteritis on APCTs could be assumed to be UTIs because the 22 patients had fever (or history of fever), pyuria or positive urine culture, elevated serum infection marker (CRP or PCT), and no other findings that could be the cause of fever or infection rather than ureteritis (Fig. 4).
The second most common cause of ureteritis observed on APCT was ureteral stone, which accounted for up to 10% of 422 ureteritis patients. Among the 42 cases of ureteritis caused by ureteral stones, 32 (76%) were presumed to be due to simple obstructive uropathy (no pyuria and no serum infection marker elevation). Additionally, 10 cases (24%) had combined UTIs (pyuria or positive urine culture and the serum infection marker elevation) along with obstruction (Fig. 4).
UTIs and ureteral stones accounted for 93% of ureteritis on APCTs conducted in the ED. Other rare causes of ureteritis were the spread of malignant tumors or nearby inflammation (Fig. 4).
Table 2 shows the clinical features and laboratory test results of 351 patients with ureteritis caused by UTIs. The median age of patients was 63 years, and more than 85% had fevers or chills. Infection markers (CRP and PCT) were elevated, and pyuria was present in more than 85% of patients (Table 2).
Table 3 shows the characteristics of 22 patients who had ureteritis caused by UTIs, but exhibited only ureteritis on the APCT (no accompanied findings of APN nor cystitis on APCTs). Of the 22 patients with only ureteritis on the APCTs, all (100%) exhibited fevers and chills at the time of their visit to the ED. However, only 36.4% had costovertebral angle tenderness and less than 50% of the patients complained of upper UTI symptoms such as flank pain and lower UTI symptoms such as dysuria, frequency, or residual urine sense.
Table 4 shows the clinical features and laboratory test results of 42 patients with ureteritis caused by ureteral stones. The median age of the patients was 55 years, and approximately 55% exhibited flank pain. Infection markers (CRP and PCT) were not elevated, and hematuria was present in about 55% of the patients.
DISCUSSIONThis is the first report on the causes and clinical features of ureteritis observed on APCTs conducted in the ED. Ureteritis was observed in approximately 6% of the 7,500 APCTs conducted in the ED during the study period. The most common cause of ureteritis was UTI, resulting in 83% of ureteritis cases. This result agrees with a previous report in which cases of ureteritis were caused by ascending infections of cystitis or descending infections of pyelonephritis by UTI causing bacteria (Escherichia coli, Staphylococci, Streptococci, Enterococci, and Proteus) rather than a primary lesion of the ureter [12]. Bacterial endotoxins cause functional changes such as decreased muscle tone and abnormal peristalsis of the ureter, and size changes such as swelling of the mucosal and submucosal layers [1,9].
The result that drew attention in this study was a small group of patients where only ureteritis was observed without pyelonephritis or cystitis. An interesting feature is that 100% of the patients had fevers upon visiting the ED, but less than 40% had presumptive symptoms or signs of UTI, while one-quarter of the patients had no pyuria. In patients with only ureteritis, it can be difficult to suspect a UTI as the cause of the fever during the initial evaluation of the patient in the ED. Therefore, for patients whose cause of fever is unclear, APCTs should actively be considered. It is necessary to examine the presence of ureteritis in APCTs carefully. In fact, the trigger for initiating this study was experience with patients with UTIs who had ureteritis without pyelonephritis or cystitis on APCTs that were conducted in search of the fever source.
The difference between this studyŌĆÖs results and those of previous studies is that ureteral stones were the second most common cause of ureteritis in ED patients. To the best of our knowledge, considering the absence of previous ED-based studies, we believe that this is the first study to report that ureteral stones are the cause of ureteritis in approximately 10% of APCTs performed in the ED. However, the mechanism of ureteritis caused by ureteral stones remains unclear. There is no study suggesting its pathophysiology and the mechanism of ureteritis may be the result of simple edema due to obstruction or inflammation caused by combined UTIs and obstruction. In this study, one-fourth of patients with ureteral stones and ureteritis exhibited positive pyuria or urine cultures, and infection-related markers increased in blood tests, suggesting that combined UTIs, as well as simple obstruction, may cause ureteritis. Alternatively, the remaining three quarters of the patients did not exhibit pyuria, positive urine cultures, and blood infection-related marker elevations. Therefore, ureteral edema due to simple obstruction could be the main cause of ureteritis. However, when ureteritis is observed in patients with ureteral stones, the possibility of subclinical infectious ureteritis cannot be excluded.
Among the APCT findings that occur in the ureter related to ureteral stones, soft-tissue rim signs (rim signs) or ureteral wall thickness should be distinguished from ureteritis [13-15]. The rim sign is an edema of the short segment ureter surrounding the ureteral stone, which is observed as a soft-tissue density on APCTs and can be used to distinguish a ureteral stone from a phlebolith [13,14]. Ureteral wall thickness is caused by localized short segment ureteral wall edema around an impacted ureteral stone. It has been reported that if the thickness of the ureteral wall surrounding the ureteral stone is 2.7 mm or greater, the possibility of spontaneous passage of the ureteral stone is very low [15]. Alternatively, ureteritis is not a local edema of the short segment ureter, but APCT findings revealed that the ureteral wall thickens by 1 mm or more, and the contrast of the ureter wall is increased over the long segment of the ureter. However, the results of this study suggest that not only infectious inflammation, but also ureteral wall edema due to obstruction can be a major cause of ureteritis. Further research is required to determine the mechanism of ureteritis, observed only in some patients with ureteral stones, and its clinical significance.
This studyŌĆÖs limitations are as follows. First, there is a high possibility that selection bias occurred as study subjects were recruited only among patients who underwent APCTs in a single-center ED. CT is not a gold standard for the diagnosis of UTIs, and there is a report that approximately 12.9% of APN patients exhibited normal CT findings [16]. However, since ureteritis is a finding that cannot be confirmed without APCT, it is inevitable to recruit ureteritis patient only from those who have undergone APCT. Second, there is a limit to the reliability of the data because the clinical parameters used are obtained through medical record research, an unavoidable fundamental limitation of retrospective research. Third, we could not explain whether the ureteritis mechanism caused by ureteral stones is due to ureteral wall edema caused by obstruction or infectious inflammation by accompanying UTIs. Lastly, we could not explain why some trauma patients exhibited ureteritis on APCTs.
In conclusion, ureteritis was a common finding observed in 6% of patients who underwent APCTs in the ED. The most common cause of ureteritis was UTI. Ureteritis can be an infection source even without symptoms or signs suggestive of UTIs. For patients whose cause of fever is unclear, APCTs should be actively considered, and it is necessary to carefully examine the presence of ureteritis in APCTs. The second major cause of ureteritis was ureteral stones that can cause ureteritis either by obstruction or combined infectious inflammation.
REFERENCES1. Bechtold RB, Chen MY, Dyer RB, Zagoria RJ. CT of the ureteral wall. AJR Am J Roentgenol 1998; 170:1283-9.
3. Kenney PJ, Stanley RJ. Computed tomography of ureteral tumors. J Comput Assist Tomogr 1987; 11:102-7.
4. Marincek B, Scheidegger JR, Studer UE, Kraft R. Metastatic disease of the ureter: patterns of tumoral spread and radiologic findings. Abdom Imaging 1993; 18:88-94.
5. Wang ZJ, Meng MV, Yeh BM, Goldstein RB. Ureteral fibroepithelial polyp. J Ultrasound Med 2008; 27:1647-9.
6. Ordon M, Ray AA, DŌĆÖA Honey RJ. Ureteritis cystica: a rare cause of ureteral obstruction. J Endourol 2010; 24:1391-3.
7. Wang LJ, Wong YC, Chen CJ, Lim KE. CT features of genitourinary tuberculosis. J Comput Assist Tomogr 1997; 21:254-8.
8. Merrimen JL, Alkhudair WK, Gupta R. Localized amyloidosis of the urinary tract: case series of nine patients. Urology 2006; 67:904-9.
9. Spataro RF. Inflammatory conditions of the renal pelvis and ureter; In: Pollack HM, editor. Clinical urography. Vol. 1. Philadelphia, PA: Saunders; 1990. p.884-901.
10. Marx M, Bettmann MA, Bridge S, Brodsky G, Boxt LM, Richie JP. The effects of various indwelling ureteral catheter materials on the normal canine ureter. J Urol 1988; 139:180-5.
11. World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997; 277:925-6.
12. Giambroni L, Monticelli L, Simeone C, Frego E. Ureteritis. Arch Ital Urol Nefrol Androl 1993; 65:31-3.
14. Heneghan JP, Dalrymple NC, Verga M, Rosenfield AT, Smith RC. Soft-tissue ŌĆ£rimŌĆØ sign in the diagnosis of ureteral calculi with use of unenhanced helical CT. Radiology 1997; 202:709-11.
15. Yoshida T, Inoue T, Taguchi M, Omura N, Kinoshita H, Matsuda T. Ureteral wall thickness as a significant factor in predicting spontaneous passage of ureteral stones of Ōēż 10 mm: a preliminary report. World J Urol 2019; 37:913-9.
16. Lee BG, Kim JW, Lee KR, et al. The clinical usefulness of computed tomography findings as a prognostic factor for patients with acute pyelonephritis in emergency department. J Korean Soc Emerg Med 2018; 29:259-66.
Fig.┬Ā2.A case of ureteritis due to urinary tract infection. The patient was a 38-year-old female with right lower quadrant area pain and fever. Diffuse urothelial wall thickening and urothelial enhancement are observed in the right ureter (arrows). 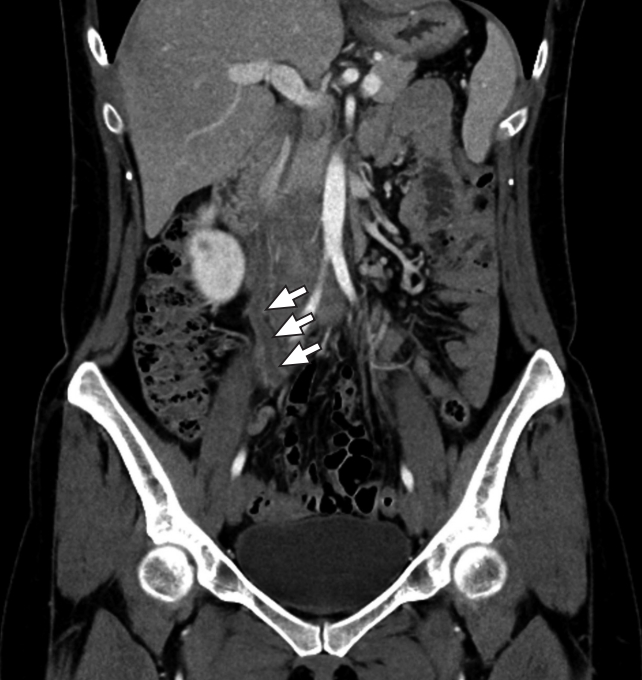
Fig.┬Ā3.A case of ureteritis associated with urinary tract stones. The patient was a 68-year-old female with abdominal pain and a ureter stone (arrowhead). The resulting obstructive uropathy are observed. Mild wall thickening of the left upper ureter (arrows) suggests combined inflammation. 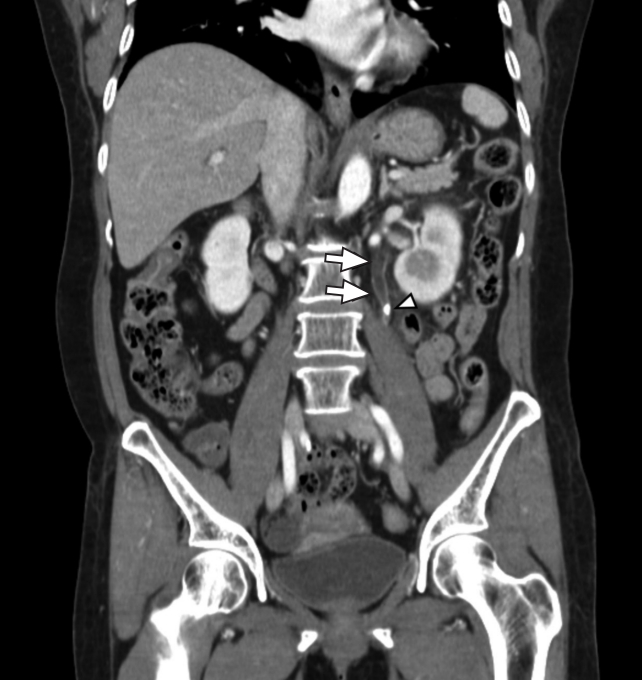
Fig.┬Ā4.Classification by etiology in 422 cases with ureteritis confirmed on intravenous contrast-enhanced abdominopelvic computed tomography. a)Ureteritis+no cause of infection other than pyelonephritis or cystitis on abdominopelvic computed tomography+fever (or history of fever)+serum infection marker (C-reactive protein or procalcitonin) elevation. b)Ureteritis+ureteric stone+no pyuria+no serum infection marker elevation. c)Ureteritis+ureteric stone+pyuria or positive urine culture+serum infection marker elevation. APN, acute pyelonephritis. 
Table┬Ā1.Primary reason for contrast-enhanced abdominal computed tomography (n=7,386)
Table┬Ā2.Clinical and laboratory characteristics of 351 patients with ureteritis caused by UTI Table┬Ā3.Clinical and laboratory characteristics of 22 patients who had ureteritis caused by urinary tract infection but showed only ureteritis on abdominopelvic computed tomography Table┬Ā4.Clinical and laboratory characteristics of 42 patients with ureteritis caused by ureteral stone |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||