Effect of rapid influenza diagnostic tests on patient management in an emergency department
Article information
Abstract
Objective
We evaluated the effect of rapid influenza diagnostic tests (RIDTs) on patient management in an emergency department for 3 years after 2009, and also identified factors associated with the choice of treatment for patients with influenza-like illnesses.
Methods
The study period consisted of three influenza epidemic seasons. Patients older than 15 years who underwent RIDTs in the emergency department and were then discharged without admission were included.
Results
A total of 453 patients were enrolled, 114 of whom had positive RIDT results and 339 had negative results. Antiviral medication was prescribed to 103 patients (90.4%) who had positive RIDT results, while 1 patient (0.3%) who tested negative was treated with antivirals (P<0.001). Conservative care was administered to 11 RIDT-positive patients (9.6%) and 244 RIDT-negative patients (72.0%) (P<0.001). Symptom onset in less than 48 hours, being older than 65 years, and the presence of comorbidities were not associated with the administration of antiviral therapy.
Conclusion
RIDT results had a critical effect on physician decision-making regarding antiviral treatment for patients with influenza-like illnesses in the emergency department. However, symptom onset in less than 48 hours, old age, and comorbidities, which are all indications for antiviral therapy, were not found to influence the administration of antiviral treatment.
INTRODUCTION
The influenza virus, which is prevalent each year due to antigenic shift and drift, is an important cause of acute respiratory infection in all age groups [1]. In 2009, due to an instance of antigenic shift, influenza A (H1N1) caused a worldwide pandemic outbreak in 214 countries, resulting in more than 18,306 deaths [2,3]. After the emergence of influenza A (H1N1), a marked increase was noted in the number of patients visiting the emergency department (ED) with suspected influenza infections.
It has become increasingly important to detect influenza rapidly in the ED, as appropriate treatment during the early stages of clinical symptoms can prevent complications and the spread of infection. Various diagnostic tools are used to detect influenza in clinical settings, although most definitive tests require a significant amount of time [4,5]. In contrast, rapid influenza diagnostic tests (RIDTs) are relatively simple, inexpensive, and convenient tests that provide point-of-care results in 10 to 30 minutes. RIDTs are now preferred over other tests in the ED, as emergency physicians are under pressure to rapidly diagnose influenza. However, the implementation of RIDTs poses some concerns. In particular, it has been reported that a certain degree of caution is necessary in interpreting the results of RIDTs and using them in treatment planning for patients with influenza-like illnesses (ILIs) [6-8].
Little research on the effects of RIDTs on the management of adult patients with ILIs in the ED has been conducted after 2009. In this study, we investigated the effect of RIDTs on the management of patients presenting with ILIs in the ED for 3 years after 2009. We also identified whether patients with ILIs in the ED are treated according to treatment guidelines.
METHODS
Study design and setting
This study was conducted in the ED of a tertiary suburban academic hospital serving 45,000 persons annually. Data on the enrolled patients were collected retrospectively from their electronic medical records by an experienced research nurse, and we performed an analysis of RIDT results and treatment modalities.
Methods
The study period consisted of 3 epidemic influenza seasons: October 2010 to March 2011, October 2011 to March 2012, and October 2012 to March 2013. RIDTs were performed at the discretion of the emergency physician without written guidance in patients who showed flu-like symptoms, respiratory symptoms, or a fever of uncertain cause during the study period. RIDTs were performed using nasopharyngeal swabs with the Genedia influenza Ag (Green Cross Corp., Yongin, Korea) in the early period of the study (2010 to 2011) and the SD Bioline rapid influenza kit (Standard Diagnostics Inc., Yongin, Korea) from 2011 to 2013 [9].
All patients older than 15 years who underwent an RIDT in the ED before being discharged without admission were included in this study, while patients who were admitted, were transferred, or died were excluded. Through a retrospective chart review by an attending physician in the ED, patients whose final diagnosis was unlikely to be an acute respiratory tract infection were excluded from the study. Data on the following variables were collected: vital signs, comorbidities, symptoms, onset of symptoms, performance of chest X-rays, complete blood count, urinalysis, blood cultures, total time spent in the ED, and RIDT results. The treatment modality was classified as antiviral, antibiotic, or conservative treatment.
ILIs were defined as a fever greater than 37.8°C with one or more respiratory symptoms in the absence of other causes [10]. We defined comorbidities as chronic medical conditions, such as asthma, chronic lung disease, heart disease, kidney disorders, endocrine disorders, a weakened immune system, and other similar conditions [10].
Statistical analyses
All continuous data were non-parametrically distributed and are presented as mean values with standard deviation. Student’s t-test for continuous variables was applied for comparisons between the two groups. Categorical variables are presented as frequencies with percentages, and were compared using the chi-squared or Fisher exact tests. Multinomial logistic regression was used to identify factors affecting the choice of treatment, using conservative management as the reference group. Data were analyzed using IBM SPSS Statistics ver. 20 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate statistical significance.
Ethical statement
The institutional review board of Korea University Ansan Hospital for clinical research approved the use of medical records for this study (AS13155). The informed consent was waived by the board.
RESULTS
In the three time intervals contained within the study period, 3008 patients were tested for influenza using RIDTs, 2,555 of whom were excluded from this study. Among the excluded patients, 1,190 were younger than 15 years and 1,123 were admitted, were transferred, or died. Among the 695 eligible patients, 242 were excluded due to a lack of ILI symptoms. Finally, a total of 453 patients were included in the study (Fig. 1). Among the 453 study patients, 114 had positive RIDT results, while 339 had negative results.

Schematic flowchart of patient inclusion in the study. RIDT, rapid influenza diagnostic test; ILI, influenza-like illness.
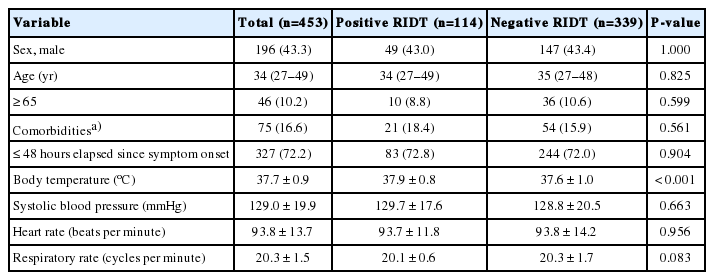
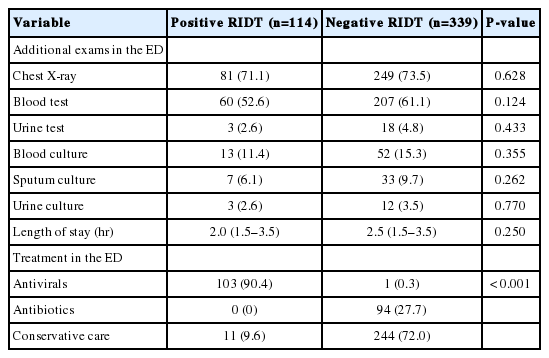
The study patients were divided into RIDT-positive and -negative groups. The two groups did not differ significantly in terms of demographics, such as sex, age, comorbidities, and time since the symptom onset (Table 1). Results of additional examinations, such as chest X-rays, blood tests, urine tests, and cultures, did not significantly differ between the two groups (Table 2). No significant difference was found between the two groups regarding the length of stay in the ED (P=0.250). Antiviral medication was prescribed to 103 patients (90.4%) who had positive RIDT results, but only 1 patient (0.3%) in the RIDT-negative group was treated with antivirals (P<0.001). Antibiotics were not prescribed to any RIDT-positive patients (0%) and were prescribed to 94 RIDT-negative patients (27.7%). Conservative treatment was administered to 11 RIDT-positive patients (9.6%) and 244 RIDT-negative patients (72.0%) (P<0.001).
Multinomial logistic analysis with conservative care as the reference group was used to investigate the association between indications for antiviral and actual treatment (Table 3). Presentation less than 48 hours after the onset of symptoms, being older than 65 years, and the presence of comorbidities, which are all indications for antiviral treatment, were included in the multinomial regression model as covariates. These variables were not found to be associated with antiviral therapy (adjusted odds ratio [aOR], 0.338; 95% confidence interval [CI], 0.074 to 2.023; aOR, 2.089; 95% CI, 0.067 to 64.729; aOR, 3.729; 95% CI, 0.268 to 51.947; respectively). In contrast, old age and presentation less than 48 hours after the onset of symptoms were significantly associated with antibiotic treatment (aOR, 2.096; 95% CI, 1.010 to 4.351; aOR, 1.492; 95% CI, 0.788 to 2.825; respectively).
DISCUSSION
In the present retrospective study, we found that most patients with positive RIDT results received antiviral treatment, while only 9.6% of RIDT-positive patients received conservative care. Additionally, multinomial logistic regression showed that elapsed time since symptom onset, age, and the presence of comorbidities were not associated with antiviral therapy in patients with ILIs. These results indicate that emergency physicians were completely reliant on RIDT results alone for decision-making about patient management.
Most healthy people infected with the influenza virus recover spontaneously without special treatment, which is referred to as conservative care, but some patients experience influenza-associated complications, such as pneumonia, which is associated with a considerable socioeconomic burden [11]. The timely detection of influenza infections and initiation of treatment reduce the burden associated with the disease, and also prevent the spread of infection [12-15]. However, diagnosing influenza based on clinical symptoms is challenging because many other respiratory viruses produce similar symptoms [16]. Therefore, appropriate laboratory tests in patients with a suspicious clinical presentation are mandatory to ensure the early and accurate diagnosis of influenza.
Most definitive influenza testing methods require a significant amount of time, as well as specialized equipment and trained operators. RIDTs, in contrast, are simple tests, require minimal training, and the results are confirmed immediately [4]. Currently, the use of RIDTs is recommended by the World Health Organization [5]. The use of appropriate RIDTs during influenza epidemics may facilitate the appropriate prescription of antivirals, as well as potentially reduce the number of additional tests ordered in the ED, the amount of antibiotics prescribed, and the length of hospital stays [17-20].
An RIDT alone, however, is insufficient to confirm influenza infection. Sensitivity and specificity analyses have been performed on various RIDTs, and a meta-analysis has reported a sensitivity of 62.3% (95% CI, 57.9% to 66.6%) compared with reverse transcription polymerase chain reaction [21-24]. Additionally, even for a single RIDT, different populations and different samples can lead to different sensitivities [15,25]. Caution must be heeded when interpreting RIDT results due to their inconsistent accuracy; while influenza can be considered, it cannot be excluded based on RIDT results alone, and emergency physicians or primary physicians caring for patients with ILIs should not base decisions regarding antiviral prescription solely on RIDT results.
Most patients with negative RIDT results in this study did not receive antiviral treatment. Negative RIDT results have a reasonable likelihood of being false negative. If an RIDT yields a negative result in a patient with an ILI who is old or has a high-risk medical condition, a clinician should not exclude influenza and defer the initiation of antiviral therapy in accordance with his or her clinical judgment. Based on the limitations of laboratory diagnostic tests, even if RIDT results are negative, recent guidelines for influenza management recommend that antivirals be administered as treatment in high-risk patients who have a higher risk of serious influenza complications [13,26].
Most otherwise healthy people who become sick with influenza do not require antiviral drugs, as most influenza infections are self-limiting. Antiviral therapy is most effective when administered early in the course of illness, within 48 hours after the onset of symptoms. Additionally, antiviral agents are associated with frequent side effects. The risk-to-benefit ratio of medications considering the time elapsed after symptom onset, in addition to the RIDT results, should be considered in the decision to initiate antiviral therapy.
Additionally, unlike past studies, our analysis did not reveal a difference in the length of stay in the ED or the performance of additional testing according to RIDT results [18,20]. This is most likely due to the fact that this study involved only adult patients discharged from the ED, in whom the symptoms were mild to moderate.
This retrospective study has several limitations. First, it was based on 3 years of medical records from one hospital, which may not be representative of all Korean hospitals, thereby reducing the generalizability of our findings. Second, this study may have some degree of selection bias, as it included patients who underwent RIDTs at the emergency physician’s discretion. Another limitation is that relatively few patients were enrolled in the study. Nonetheless, significant differences were found between the RIDT-positive and RIDT-negative groups in terms of treatment strategies. Finally, we did not consider local influenza prevalence during these seasons. RIDTs are of limited use when the local influenza prevalence is low [14]. The prevalence of influenza varies between and within seasons, which can affect how physicians interpret RIDT results.
The present study suggests that RIDT results had a major effect on physician decision-making regarding the initiation of antiviral treatment for patients with ILIs in the ED. However, symptom onset within 48 hours of presentation, old age, and the presence of comorbidities were not found to influence the use of antivirals. Although RIDTs have several advantages, careful clinical evaluations in conjunction with RIDT results are necessary to ensure that patients with ILIs receive appropriate clinical care.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
Emergency physicians manage large numbers of patients with influenza-like illnesses every endemic season.
What is new in the current study
Rapid influenza diagnostic tests had a critical effect on physician decision-making regarding antiviral treatment for patients with influenza-like illness in the emergency department. However, indications for antiviral therapy were not found to influence the administration of antiviral treatment.