Brain computed tomography angiography in postcardiac arrest patients and neurologic outcome
Article information
Abstract
Objective
This study aimed to analyze intracranial vessels using brain computed tomography angiography (CTA) and scoring systems to diagnose brain death and predict poor neurologic outcomes of postcardiac arrest patients.
Methods
Initial brain CTA images of postcardiac arrest patients were analyzed using scoring systems to determine a lack of opacification and diagnose brain death. The primary outcome was poor neurologic outcome, which was defined as cerebral performance category score 3 to 5. The frequency, sensitivity, specificity, positive predictive value, negative predictive value, and area under receiver operating characteristic curve for the lack of opacification of each vessel and for each scoring system used to predict poor neurologic outcomes were determined.
Results
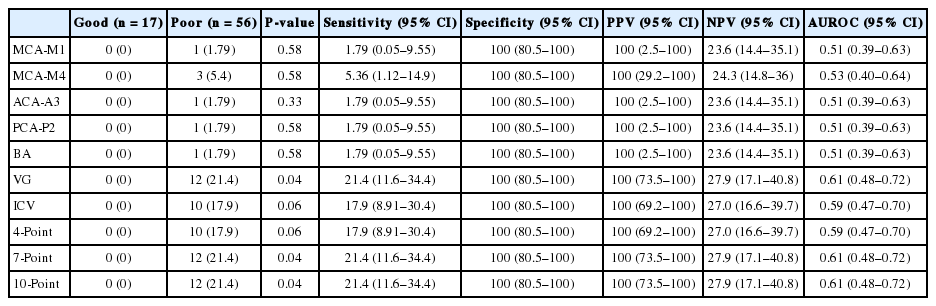
Patients with poor neurologic outcomes lacked opacification of the intracranial vessels, most commonly in the vein of Galen, both internal cerebral veins, and the mid cerebral artery (M4). The 7-score results (P=0.04) and 10-score results were significantly different (P=0.04) between outcome groups, with an area under receiver operating characteristic of 0.61 (range, 0.48 to 0.72). The lack of opacification of each intracranial vessel and all scoring systems exhibited high specificity (100%) and positive predictive values (100%) for predicting poor neurologic outcomes.
Conclusion
Lack of opacification of vessels on brain CTA exhibited high specificity for predicting poor neurologic outcomes of patients after cardiac arrest.
INTRODUCTION
Predicting the outcomes of postcardiac arrest patients has been of great interest because this information can be beneficial to families and treating physicians [1-3]. Although most prognostic factors predict outcomes 72 hours or more after cardiac arrest [3-5], initial brain imaging with computed tomography (CT) can provide information concerning the severity of brain injury earlier during the course of treatment. Recent studies have focused on predicting outcomes by analyzing grey-to-white matter ratios or optic nerve sheath diameters, which have shown various prognostic capabilities [6-9].
Brain CT angiography (CTA) is noninvasive, readily available, and beneficial for diagnosing many intracranial vascular abnormalities. This modality is used in our emergency department for patients with sudden cardiac arrest and the return of spontaneous circulation (ROSC) to diagnose a cerebral aneurysm, which is not uncommon in Asian populations [10], intracranial vascular abnormalities, or vascular injury during hanging asphyxial arrest.
Multiple studies have utilized CTA for diagnosing brain death. Three evaluation systems, the 10-score scale, 7-score scale, and 4-score scale, which investigate 10, 7, and 4 specific intracranial vessels, have been used. One point is given for each vessel lacking opacification, and brain death is diagnosed if analyzed vessels are not opaque [11-13].
CTA findings of postcardiac arrest patients have not been studied. Therefore, we aimed to retrospectively analyze vessels using CTA and scoring systems to diagnose brain death and predict poor neurologic outcomes of postcardiac arrest patients.
METHODS
This study was approved by the institutional review board of Ajou University Medical Center (AJIRB-MED-MDB-18-165). Informed consent was waived for this study due to its retrospective nature.
Study design and population
Medical records and brain CTA images of out-of-hospital cardiac arrest survivors from a single regional tertiary care center from January 2012 to June 2017 were retrospectively reviewed and analyzed. Postcardiac arrest patients who underwent targeted temperature management (TTM) and were at least 18 years of age were included in the analysis. Patients who did not undergo brain CTA evaluation, who underwent brain CTA 6 hours after ROSC, and who had previous intracranial lesions or other conditions that affected the interpretation of brain CTA images were excluded from the study.
Postcardiac arrest care and TTM
Approximately 87,000 patients visit the emergency department of our institute annually. Patients who achieve ROSC after out-of-hospital cardiac arrest are evaluated if they can respond to commands. If patients are not responsive, then they are evaluated with brain CT to exclude cerebral hemorrhage as the cause of arrest. Some patients are evaluated with brain CTA to diagnose acute intracranial and extracranial vascular abnormalities based on the medical history and the decision of the treating physician. Patients who are qualified undergo TTM treatment according to a standardized protocol. Our postcardiac arrest protocol recommends a target temperature of 33°C. If the patient is hemodynamically unstable, has bleeding tendencies, or has a severe infection, then a higher target temperature could be chosen by the treating physician. TTM is performed using temperature-managing devices with a feedback loop system (Artic Sun Energy Transfer Pads, Medivance Corp, Louisville, CO, USA; or Cool guard Alsius Icy Heat Exchange Catheter, Alsius Corporation, Irvine, CA, USA). All patients underwent sufficient sedation and analgesia and shivering control and seizure control if needed.
Data collection
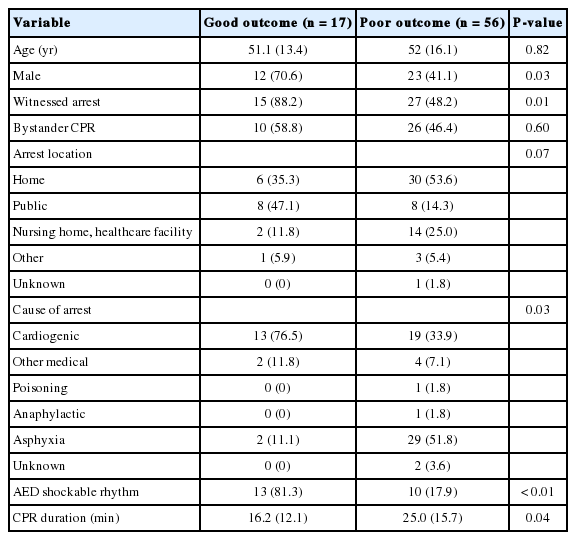
Demographic data (such as age and sex), cardiopulmonary resuscitation (CPR) data (such as witnessed arrest, bystander CPR, location of arrest, cause of arrest, initial rhythm, automatic external defibrillator shock), and patient outcomes expressed as the cerebral performance category (CPC) score were obtained from the electronic medical records. CPR duration was defined as the sum of the duration of CPR, both prehospital and in-hospital. Neurologic outcome, which was the primary outcome of the study, was assessed using the CPC at 1 month. A good neurologic outcome was defined as CPC 1 or 2, and a poor neurologic outcome was defined as CPC 3 to 5.
Brain CTA protocol and analysis and scoring systems
All patients underwent brain CTA using a 128-channel scanner (SOMATOM Definition Edge Siemens, Erlangen, Germany) or 16- channel scanner (SOMATOM Sensation 16, Siemens) with the following parameters: tube voltage, 100 kV; tube current, 178 mAs; slice/increment, 0.8 mm/0.4 mm; collimation, 128×0.625; rotation time, 0.5 seconds; pitch, 0.67; and matrix, 512 for the 128- channel scanner; 100 kV, 170 mAs, 1 mm/0.5 mm, 16×0.75, 0.5 seconds, 0.75, and 512 for the 16-channel scanner. Injections of 1.2 mL/kg of an iodinated contrast agent, ioversol (Optiray 320, Mallinckrodt, Hazelwood, MO, USA) up to a maximum of 90 mL were administered at a rate of 4 mL/sec and immediately followed by a saline bolus of 15 mL. CTA source images were obtained by the bolus triggering method; the common carotid artery (C-spine 6–7 level) was selected as the trigger location. The contrast bolus injected in the region of interest, which was the common carotid artery, reached 120 Hounsfield units. Then, the CTA source image scan was started. The CTA source images were post-processed to create coronal, sagittal, and axial multi-planar reformatting in the maximum intensity projection images and volume-rendered three-dimensional images.
Two emergency physicians blinded to patient outcomes independently reviewed the brain CTA in the picture archiving and communication system of the radiology workstation (Centricity Enterprise, GE, Chicago, IL, USA). The lack of opacity on brain CTA images of both anterior cerebral artery A3 segments, both middle cerebral artery M4 segments (MCA-M4), both posterior cerebral artery P2 segments, the basilar artery, both internal cerebral veins (ICV), and the vein of Galen (VG) were primarily observed [8-11]. We also analyzed the lack of opacity of these vessels using the 4-score, 7-score, and 10-score scoring systems that were used to diagnose brain death (Fig. 1). One point was given for each vessel without opacity. We used each scoring system to analyze the diagnostic value of the lack of opacification in each vessel.
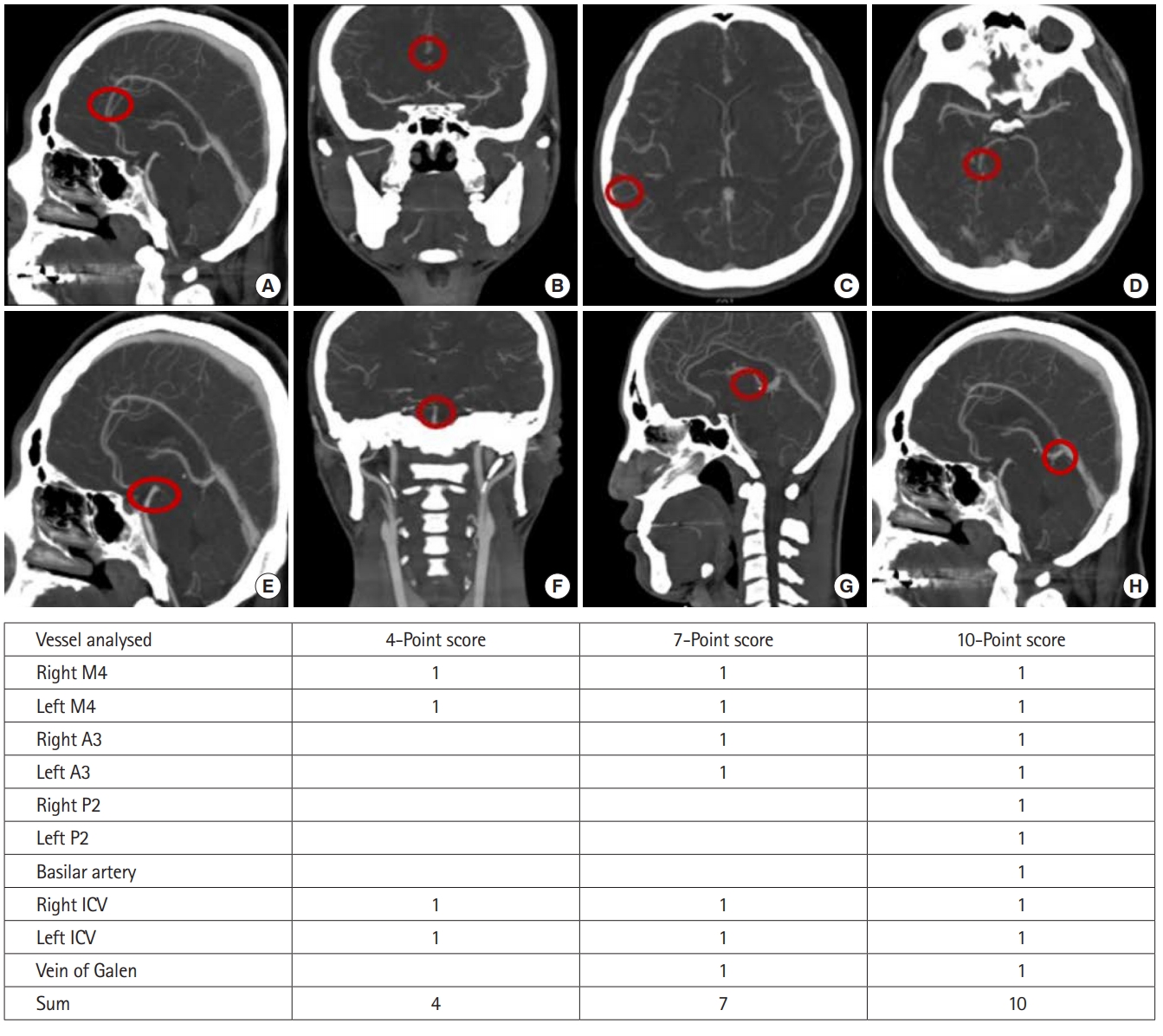
Ten vessels used for diagnosing brain death and predicting poor neurologic outcomes of postcardiac arrest patients. (A, B) Anterior cerebral artery A3 segment. (C) Middle cerebral artery M4 segment. (D) Posterior cerebral artery P2 segment. (E, F) Basilar artery. (G) Internal cerebral vein (ICV). (H) Vein of Galen. A point was given when the vessel was not opaque.
Statistical analysis
Continuous variables were expressed as the mean and standard deviation. The t-test was used for comparison of continuous variables. Categorical variables were expressed as percentages, and the chi-square test was used for comparison. P<0.05 was considered statistically significant. Interobserver agreement of brain CTA measurement was calculated by using kappa statistics. Diagnostic values (sensitivity, specificity, positive predictive value, negative predictive value, area under the receiver operating characteristic curve) were used to measure the neurologic outcome associated with each vessel and whether there was a lack of opacification in at least one vessel according to the 4-score scale, 7-score scale, and 10-score scale. We used Stata ver. 15.0 (StataCorp, College Station, TX, USA) for statistical analyses.
RESULTS
Baseline patient characteristics
A total of 73 patient images were analyzed (poor neurologic outcome, n=56) (Fig. 2). Baseline characteristics are shown in Table 1. Age (P=0.82) and location of arrest (P=0.07) did not differ between groups. However, the good neurologic outcome group had more male patients (12 [70.6%] females vs. 23 [41.1%] males, P=0.03), more automatic external defibrillator shockable rhythms (13 [81.3%] vs. 10 [17.9%], P<0.01), and shorter CPR durations (16.2 [12.1%] vs. 25.0 [15.7%] minutes, P=0.04) compared to the poor neurologic outcome group. The good and poor neurologic outcome groups had different causes of arrest (P=0.03). Asphyxia was the most common cause of arrest in the poor neurologic outcome group (29 [51.8%]), and a cardiogenic cause of arrest was most common in the good neurologic outcome group (13 [76.5%]). A higher proportion of female patients experienced asphyxia, resulting in sex differences between groups.

Study flow. OHCA, out-of-hospital cardiac arrest; TTM, targeted temperature management; CTA, computed tomography angiography; ROSC, return of spontaneous circulation.
Brain CTA analysis and neurologic outcomes
The results of brain CTA and the neurologic outcomes are presented in Table 2. Patients with good neurologic outcomes had opacification in all vessels analyzed in all of the brain death scoring systems. However, of 56 patients with poor neurologic outcomes, three (5.4%) patients lacked opacification in MCA-M4, 12 (21.4%) patients lacked opacification in VG, and 10 (17.9%) patients lacked opacification in both ICV. All patients in the good neurologic outcome group had a score of 0 according to all brain death assessment scores. However, 10 (17.9%) patients, 12 (21.4%) patients, and 12 (21.4%) patients lacked opacification in at least one vessel according to the 4-score scale, 7-score scale, and 10- score scale, respectively, in the poor neurologic outcome group. The 7-score (P=0.04) and 10-score (P=0.04) scoring system results for brain death were statistically different between outcome groups. The specificity and positive predictive value for predicting poor neurologic outcomes was 100% for all scoring systems and all vessels. This suggests that a lack of vessel opacification can predict poor neurologic outcomes. Although sensitivity of the vessels and scoring systems was low, the MCA-M4 (5.34%), VG (21.4%), and both ICV (17.9%) were more sensitive than other vessels. The 7-score scale and 10-score scale both had a sensitivity of 21.4% and an area under the receiver operating characteristic curve of 0.61. The kappa value between reviewers was 0.92, thus exhibiting high correlation between them.
DISCUSSION
This study sought to analyze the opacification of vessels using CTA and apply scoring systems to diagnose brain death in postcardiac arrest patients. Lack of opacification in any vessel had high specificity for predicting poor neurologic outcomes.
Many prognostic factors for postcardiac arrest patients have been studied, including neurologic examinations, electrophysiological tests such as electroencephalography or evoked potential, and biomarkers to allocate critical care resources, because they can provide important information to families and physicians [14-16]. Although these modalities are utilized later after cardiac arrest, brain imaging can be performed early after ROSC and usually before TTM. Brain CTA is a noninvasive imaging modality that can be quickly and easily performed within 24 hours. Some concerns regarding brain CTA are that the injection of the contrast agent may result in side effects such as acute kidney injury or allergic reactions, including anaphylaxis. Furthermore, brain CTA may expose the patient to a higher radiation dose than a simple routine brain CT. These disadvantages of brain CTA may restrict the general use of this modality for routine prediction of postcardiac arrest patients. Although it is not common to use brain CTA as an initial imaging method for postcardiac arrest patients, we think that this study may help guide the prediction of outcomes of postcardiac arrest patients in the rare case when a CTA image is obtained.
The findings of our study coincide with those of previous studies using scoring systems to diagnose brain death. VG was the most sensitive vessel, followed by both ICV and MCA-M4, for diagnosing poor neurologic outcomes of postcardiac arrest patients. The MCA-M4, ICV, and VG were also sensitive for diagnosing brain death [11,17]. This is thought to be due to the increased intracranial pressure, resulting in increased cerebral vascular resistance and compression of intracranial vessels [11].
There were many limitations to this study. Because this was a retrospective study of patients who had undergone brain CTA, the cause of arrest was mostly hanging asphyxia. Although this may have limited the study by generalizing the results to other causes of arrest, patients with cardiogenic arrest were also included in the study and had results that were similar to those of asphyxia arrest patients. Second, this was a single-center study with a limited number of patients. Third, the decision to perform brain CTA was made solely by the treating physician. Therefore, the tendency to perform brain CTA for certain causes of arrest, such as asphyxia, may have resulted in selection bias. Fourth, brain CTA was performed during the early phase of postcardiac arrest treatment, and physicians were not blinded to the results. This may have affected the outcomes by resulting in self-fulfilling prophecies.
In summary, vessels lacking opacification on brain CTA can predict poor neurologic outcomes of postcardiac arrest patients with high specificity. Although the sensitivity of the lack of opacification was not very high, among the vessels analyzed, the VG, both ICV, and MCA-M4 were sensitive.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
Minjung Kathy Chae and Seung Eun Lee were supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (no. 2018R1C1B60035).
References
Article information Continued
Notes
Capsule Summary
What is already known
Previous studies have investigated methods to prognosticate post cardiac arrest patients with routine brain computed tomography.
What is new in the current study
Lack of opacification of vessels in brain computed tomography angiography exhibited high specificity in predicting poor neurologic outcome in post cardiac arrest patients.