2020 Korean Guidelines for Cardiopulmonary Resuscitation. Part 4. Adult advanced life support
Article information
BACKGROUND OF THE 2020 GUIDELINES FOR ADULT ADVANCED LIFE SUPPORT
Adult advanced life support (ALS) is an important part of the chain of survival, promoting systemic circulation and oxygen supply during high-quality basic life support in patients with cardiac arrest. It includes advanced airway management, manual defibrillation, pharmacological therapy with vasopressors and antiarrhythmic agents, use of an extracorporeal circulation device, and other techniques to achieve return of spontaneous circulation (ROSC) [1-3].
Several milestone studies on ALS have been published since the publication of the 2015 Korean cardiopulmonary resuscitation (CPR) guidelines. These include a multicenter, randomized, double-blind, placebo-controlled study evaluating the effect of epinephrine in patients with out-of-hospital cardiac arrest (OHCA) as well as a comparative study on amiodarone, lidocaine, or placebo in patients with refractory shockable rhythm [4,5]. A study comparing the use of a supraglottic airway (SGA) device and endotracheal intubation for advanced airway management during OHCA has also been published [6].
Based on new research evidence, relevant contents of the 2015 CPR guidelines were revised in the 2020 Korean Adult ALS guidelines, with the addition of recommendations such as double sequential defibrillation, application of ultrasound, and monitoring of cerebral oxygen saturation during CPR [7-12]. In particular, the establishment of a rapid response team (RRT) or medical emergency team and the application of an early warning score were emphasized for preventing in-hospital cardiac arrest (IHCA) [13-19]. Specialized guidelines for the resuscitation of patients with confirmed or suspected coronavirus disease 2019 (COVID-19) have also been included [20,21].
ADULT ALS ALGORITHMS
High-quality CPR should be performed during all processes of ALS. The defibrillation pads or electrodes should be applied to the patient to monitor the electrocardiography (ECG) rhythm immediately after the ALS team arrives. Based on the ECG rhythm analysis, a team leader should decide on a treatment strategy as soon as possible. All team members should be proficient in the entire ALS algorithm so that it can be performed efficiently.
Adult ALS for IHCA
When an ALS team arrives at the scene, the defibrillation pads or electrodes should be attached to the patient to analyze the ECG rhythm immediately. To ensure high-quality CPR, newly arrived providers should perform chest compressions and ventilation in place of basic life support (BLS) providers. The chest compression-to-ventilation ratio should be 30:2 on 100% oxygen if an advanced airway has not been placed. Cardiac arrest rhythms are classified as shockable rhythms (ventricular fibrillation [VF] and pulseless ventricular tachycardia [pVT]) and non-shockable rhythms (asystole and pulseless electrical activity [PEA]). If the cardiac rhythm shows as a pVT or PEA, ALS providers should palpate the carotid artery to confirm the absence of a pulse. Since the ECG rhythm might change during CPR, providers should analyze it every 2 minutes and follow the treatment algorithm according to the analysis (Fig. 1).
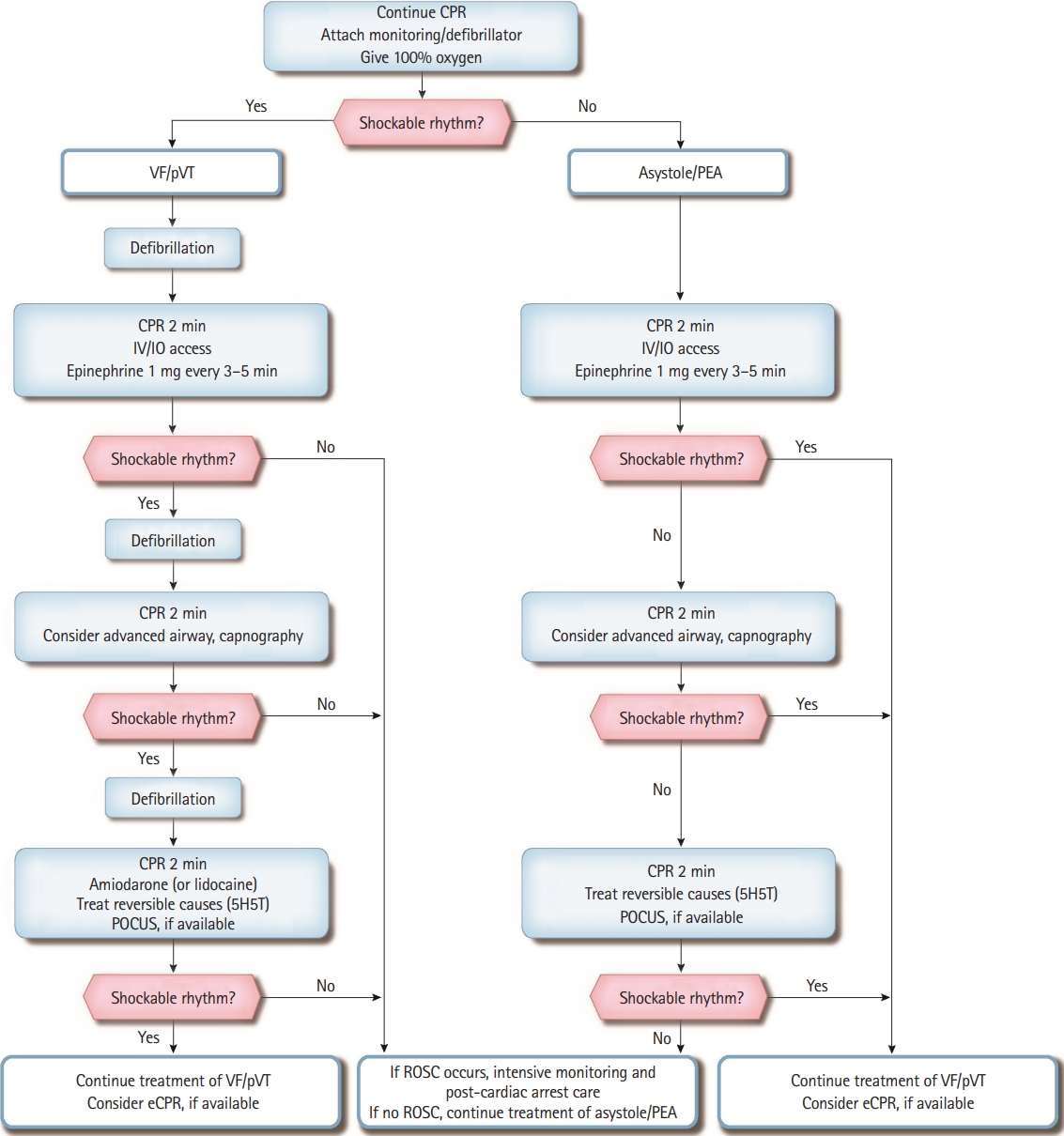
Adult advanced life support algorithm for in-hospital cardiac arrest. CPR, cardiopulmonary resuscitation; VF, ventricular fibrillation; pVT, pulseless ventricular tachycardia; IV, intravenous; IO, intraosseous; PEA, pulseless electrical activity; 5H, hypovolemia, hypoxia, hyperkalemia, hydrogen ion (acidosis), and hypothermia; 5T, tension pneumothorax, tamponade (cardiac), toxin, thrombosis (pulmonary), and thrombosis (coronary); POCUS, point-of-care ultrasound; eCPR, extracorporeal cardiopulmonary resuscitation; ROSC, return of spontaneous circulation.
Shockable rhythms (VF and pVT)
Because VF and pVT can be terminated with defibrillation, they are also known as “shockable rhythms.” Providers should deliver a single shock (biphasic wave defibrillator: 120–200 J, monophasic wave defibrillator: 360 J) when a shockable rhythm is confirmed on the ECG monitor and resume CPR immediately for 2 minutes, beginning with chest compressions without a pulse check or ECG rhythm analysis. If the provider cannot identify the manufacturer’s recommended dose of the biphasic defibrillator, defibrillation (shock) should be conducted at 200 J. After the first shock is delivered, an intravenous (IV) or intraosseous (IO) route for drug administration should be established, and a 1 mg bolus of epinephrine should be administered, followed by a bolus of IV fluids. The IO route should be attempted only if the IV access is unsuccessful or not feasible. Administration of 1 mg epinephrine should be repeated every 3 to 5 minutes while ALS continues. After 2 minutes of CPR, the ECG rhythm should be checked. If a shockable rhythm persists, the provider should deliver another shock with equivalent or higher energy than the previous defibrillation and resume CPR immediately.
Meanwhile, another provider should consider performing advanced airway management and capnography. If an advanced airway is in place, it is reasonable to deliver 1 breath every 6 seconds (10 times/min) with continuous chest compressions. If endotracheal intubation has been performed, monitoring of the end-tidal carbon dioxide (ETCO2) can assess the CPR quality and detect ROSC. If a shockable rhythm persists after 2 minutes of CPR, the provider should deliver another shock and resume CPR. Amiodarone or lidocaine can be prepared for refractory VF or pVT that is unresponsive to the second shock and may be administered immediately after the third shock. The recommended first dose is 300 mg for amiodarone or 1 to 1.5 mg/kg for lidocaine. If the VF or pVT persists, a second dose of amiodarone 150 mg or lidocaine 0.5 to 0.75 mg/kg can be administered. Based on the patient’s cardiac arrest situation or medical history, immediate identification and treatment of the reversible causes of cardiac arrest may facilitate ROSC. Point-of-care ultrasound (POCUS), if available and not interfering with CPR, may be helpful to identify the cause of cardiac arrest. Extracorporeal CPR (eCPR) may be considered if ROSC is not achieved despite a high-quality CPR and if there is a potentially reversible cause of cardiac arrest that might benefit from temporary cardiorespiratory support.
Non-shockable rhythms (asystole and PEA)
As asystole and PEA do not require defibrillation during CPR, they are referred to as “non-shockable rhythms.” Asystole is the absence of ventricular contraction regardless of the presence of atrial contraction. Meanwhile, PEA is a clinical condition defined by the absence of measurable blood pressure and palpable pulse despite the presence of sufficient electrical discharge and a heterogeneous group of pulseless rhythms, including pseudo-electromechanical dissociation, idioventricular rhythms, ventricular escape rhythms, and brady-asystolic rhythms. Patients with cardiac arrest with PEA can be treated if the reversible causes are identified and corrected, even though the survival rate of cardiac arrest patients with asystole is very low. If the ECG rhythm is nonshockable, chest compressions should be performed immediately for 2 minutes while the route (IV or IO) for drug administration is being established. Subsequently, a 1 mg bolus of epinephrine should be administered as soon as possible, along with an IV fluid push. Administration of 1 mg of epinephrine should be repeated every 3 to 5 minutes while ALS continues. After 2 minutes of CPR, the ECG rhythm should be analyzed. If the asystole or PEA persists, chest compressions should be continued, and advanced airway management should be performed. If a shockable rhythm is observed, the shock should be delivered according to the “shockable rhythm” algorithm. POCUS, if available and not interfering with CPR, can be helpful to identify the cause of cardiac arrest.
Adult ALS for OHCA
The adult ALS algorithm for OHCA is similar to that for IHCA. However, in OHCA, bag-mask ventilation (BMV) is preferably recommended for providers who are unfamiliar with advanced airway management. Using an SGA device is also preferred to endotracheal intubation for providers with little experience in endotracheal intubation. It is recommended to consider transferring the patient to the hospital after performing ALS on scene for 10 minutes if the emergency medical technicians (EMTs) participating in the resuscitation could perform ALS, including advanced airway management and epinephrine administration. In contrast, it is recommended to transfer the patient to the hospital after resuscitation for 6 minutes according to the BLS guidelines if the prehospital resuscitation team could not perform ALS. For example, in the case of a multitiered dispatch system, if the first EMT who arrives at the scene can perform ALS, the EMT should consider transferring the patient to the hospital after providing ALS for 10 minutes; otherwise, providing 6 minutes of CPR and then transferring the patient to the hospital would be appropriate. Scene time interval could be modified under direct medical oversight (Fig. 2).
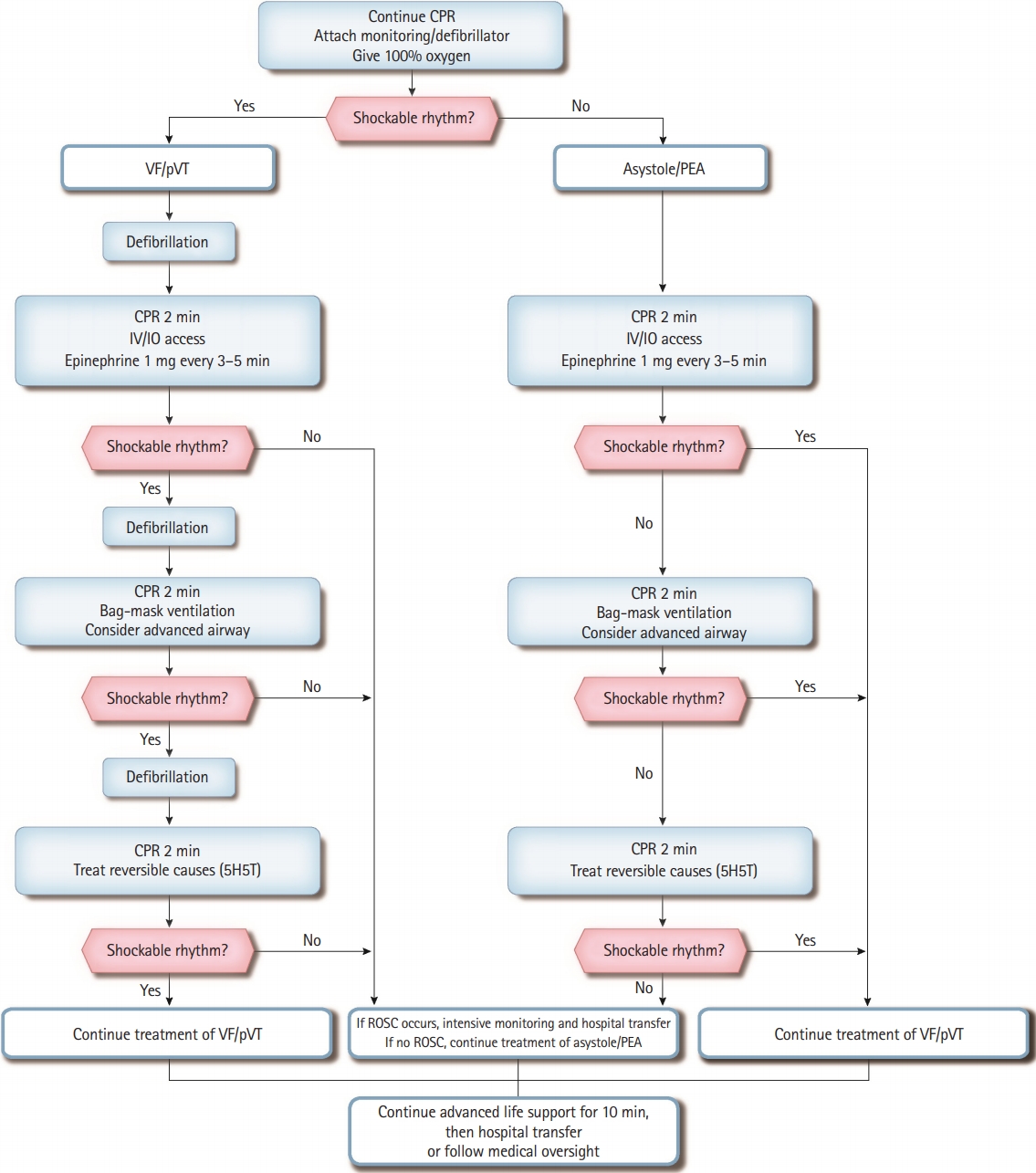
Adult advanced life support algorithm for out-of-hospital cardiac arrest. CPR, cardiopulmonary resuscitation; VF, ventricular fibrillation; pVT, pulseless ventricular tachycardia; PEA, pulseless electrical activity; IV, intravenous; IO, intraosseous; 5H, hypovolemia, hypoxia, hyperkalemia, hydrogen ion (acidosis), and hypothermia; 5T, tension pneumothorax, tamponade (cardiac), toxin, thrombosis (pulmonary), and thrombosis (coronary); ROSC, return of spontaneous circulation.
Adult ALS for cardiac arrest during the COVID-19 outbreak
Chest compressions, positive pressure ventilation, and securing an advanced airway can generate aerosols, which could increase the risk of transmission of COVID-19. It is essential that providers protect themselves and their colleagues from unnecessary exposure to both airborne and droplet particles. Members of the ALS team should wear appropriate personal protective equipment (PPE) with a minimum of Level D protection, including a mask (a minimum of N95 or FFP3) or powered air-purifying respirator, gloves, gown, and goggles or face shield before starting ALS. For patients with a poor prognosis, the provision of resuscitation should be reevaluated, and ALS should be started when resuscitation is deemed necessary. The ALS team should comprise the minimum number of essential personnel. A mechanical CPR device can be used to minimize contact with the patient. For ECG rhythm check and defibrillation, a defibrillation pad should be used, if feasible. If advanced airway management is required, the provider should stop the chest compressions, perform endotracheal intubation with a cuffed tube, and connect the endotracheal tube with a high-efficiency particulate air filter, if available. Video laryngoscopy for endotracheal intubation should also be considered to reduce exposure to aerosol. If endotracheal intubation is delayed, the provider should consider attaching an SGA device or bag-valve mask to a high-efficiency particulate air filter with a tight seal. After completion of resuscitation, PPE should be disposed of according to the infection control guidelines and ALS providers should wash their hands thoroughly with soap and water or disinfect them with alcohol-based hand sanitizer as soon as possible, followed by a change of clothes (Fig. 3).

Adult advanced life support for cardiac arrest during the COVID-19 outbreak. PPE, personal protective equipment; CPR, cardiopulmonary resuscitation; VF, ventricular fibrillation; pVT, pulseless ventricular tachycardia; PEA, pulseless electrical activity; IV, intravenous; IO, intraosseous; 5H, hypovolemia, hypoxia, hyperkalemia, hydrogen ion (acidosis), and hypothermia; 5T, tension pneumothorax, tamponade (cardiac), toxin, thrombosis (pulmonary), and thrombosis (coronary); POCUS, point-of-care ultrasound; eCPR, extracorporeal cardiopulmonary resuscitation; ROSC, return of spontaneous circulation.
RECOMMENDATIONS FOR ADULT ALS
Feedback device and monitoring during ALS
Using a feedback device and monitoring the patient can help optimize CPR quality and assess patient status during CPR [22]. However, it is not recommended to implement real time CPR feedback devices routinely because their use has no significant effect on resuscitation outcome compared with not using them at all [23-26]. Nevertheless, real time CPR feedback devices in medical systems can be used for monitoring CPR quality. No studies have proven the usefulness of checking the pulse to evaluate the effectiveness of chest compressions during CPR. However, the pulse can be checked as a method to confirm ROSC. Providers should check the pulse within 5 to 10 seconds to minimize chest compression interruption and, if there is no pulse, resume chest compressions immediately [27-29]. Advanced monitoring parameters such as ETCO2 partial pressure, coronary perfusion pressure, and central venous oxygen saturation can be used as physiological monitoring indices during ALS. During cardiac arrest, ETCO2 levels reflect the cardiac output generated by chest compressions. An ETCO2 level of <10 mmHg despite high-quality CPR of >20 minutes has been associated with an extremely poor chance of ROSC and survival outcome. An abrupt increase of ETCO2 during resuscitation may imply ROSC [30-34]. However, ETCO2 partial pressure should not be used as a sole indicator of CPR quality and ROSC.
Measuring the ETCO2 level from an SGA or bag-valve-mask airway cannot adequately predict the efficiency of chest compressions and resuscitation outcomes, but it is the most reliable method for confirming the correct placement of an endotracheal tube after intubation [35]. If the coronary perfusion pressure is <15 mmHg during CPR, the chance of ROSC is low [36]. However, coronary perfusion pressure is difficult to monitor because it is calculated from the arterial relaxation “diastolic” pressure and central venous pressure during CPR. If the central venous oxygen saturation (ScvO2) has been monitored prior to cardiac arrest, or if catheterization is possible without interfering with resuscitation, providers can consider measuring the ScvO2 to monitor CPR quality. Studies have shown that a ScvO2 of <30% during CPR is associated with failure to restore spontaneous circulation; thus, providers should ensure high-quality CPR to maintain the ScvO2 above 30% [37].
Pulse oximetry is not a suitable monitoring method because peripheral circulation is insufficient during cardiac arrest. Nevertheless, it can be used to evaluate proper oxygenation after ROSC [27]. Moreover, regional cerebral oxygen saturation monitoring using near-infrared spectroscopy during adult CPR can be easily applied noninvasively to help assess cerebral perfusion status and predict ROSC and prognosis [11,38].
Vascular access for drug administration in cardiac arrest
When performing high-quality CPR and defibrillation, providers should be able to establish a route for drug administration. This procedure should not interfere with chest compressions [39,40]. The peripheral IV route is the traditional approach; it is reasonable that providers should first attempt IV access for drug administration during CPR [41,42]. A fluid bolus of 20 mL should be injected after drug administration to promote the entry of drugs into the central circulation. Lifting the arm during or after drug injection might theoretically promote the inflow of drug into the central circulation by gravity, although there is no evidence to support this. IO access, on the other hand, is safe and useful for infusion of fluids, administration of drugs, and blood sampling in patients of any age. However, two studies have shown that the rates of ROSC and survival to hospital discharge were lower with using IO access than with using IV access during cardiac arrest [40,43]. IO access may nonetheless be considered if IV access is unsuccessful or not feasible. Another option is drug administration via a central venous catheter, which can achieve higher peak concentrations and more rapid circulation than peripheral IV administration [44-46]. Such a route may be considered by skilled providers when IV and IO access are unsuccessful or not feasible, although it may require interruption of CPR. An endotracheal tube may also be used, but drug delivery and pharmacological effects can be unpredictable with this route [47,48].
Medications during cardiac arrest
Vasopressors
The alpha-adrenergic effect of epinephrine increases coronary artery perfusion pressure and cerebral perfusion pressure during CPR. In a study comparing the effects of epinephrine and placebo in patients with OHCA, the 3-month survival rate was higher in the group administered 1 mg of epinephrine at intervals of 3 to 5 minutes [4]. Therefore, it is recommended to administer 1 mg of epinephrine at intervals of 3 to 5 minutes to patients with cardiac arrest regardless of the cardiac arrest rhythm. Other studies with a very low level of evidence showed that high-dose epinephrine administration improved spontaneous circulation and survival to hospital admission compared with standard-dose administration (1 mg every 3 to 5 minutes in the form of a 1:1,000 ampule or 1:10,000 prefilled syringe formula) but did not improve survival to hospital discharge and neurological outcomes [49,50]. Therefore, routine administration of high doses of epinephrine is not recommended; however, an expert may deem it necessary [51].
The non-adrenergic peripheral vasoconstrictor, vasopressin, can also augment arterial blood pressure. A study comparing the effects of epinephrine and vasopressin found no difference in prognosis in patients with OHCA [52,53]. No differences were also found in the resuscitation outcomes in trials comparing vasopressin alone or vasopressin combined with epinephrine to epinephrine alone in cardiac arrest patients [54,55]. However, no studies have shown that the use of vasopressin alone or in combination with epinephrine worsens the prognosis of cardiac arrest patients [52-56]. Therefore, 40 units of vasopressin or vasopressin in combination with epinephrine may be administered as an alternative for the first or second dose of epinephrine.
Antiarrhythmic drugs
Administration of amiodarone or lidocaine to OHCA patients with refractory VF or pVT improved survival to hospital admission but did not improve overall survival to hospital discharge or survival with good neurological outcome. The critical outcome of survival to hospital discharge was improved with amiodarone or lidocaine compared to placebo in patients with bystander-witnessed cardiac arrest; however, there was no difference between amiodarone and lidocaine with regard to ROSC, favorable neurological outcome, or survival to hospital discharge [5]. Additionally, the routine use of magnesium is not recommended in adult cardiac arrest patients [57]. When using amiodarone, the first dose is 300 mg IV or IO, and 150 mg can be added if the patient remains unresponsive. The first dose of lidocaine is 1 to 1.5 mg/kg IV or IO bolus, and then 0.5 to 0.75 mg/kg every 5 to 10 minutes; up to 3 mg/kg can be added [57,58]. Although lidocaine or beta-blockers may be considered for the prevention of arrhythmias immediately after ROSC in patients with refractory VF or pVT, their effectiveness remains uncertain [59,60].
Defibrillation
Early defibrillation is crucial in patients with VF or pVT. Therefore, providers should deliver a shock immediately when the defibrillator is ready and a shockable rhythm is detected on ECG [61-65]. Biphasic waveform defibrillators are preferred over monophasic defibrillators because they are more safe and effective [66,67]. In unmonitored cardiac arrest, a single-shock strategy is suggested because the success rate of the first shock is around 90% and chest compression interruption is prolonged in stacked shocks [68,69]. Chest compressions should be performed even while the defibrillator is charging. The chest compression interruption before and after defibrillation should be within 10 s during the entire process of resuscitation [70-73]. For effective defibrillation, the shock should be delivered at the end of expiration to decrease transthoracic resistance, and it would be better to use defibrillation pads rather than paddles to ensure contact with the chest pads [74-76]. The usefulness of double sequential defibrillation for refractory VF or pVT has not been established [9,77].
Advanced airway management for resuscitation
Providers may use either BMV or an advanced airway strategy during ALS. However, in the case of advanced airway management in OHCA, EMTs without adequate training and experience in endotracheal intubation should use an SGA device. It is recommended that only experienced EMTs should try to perform endotracheal intubation [78-83]. For adult patients with IHCA, either an SGA device or endotracheal intubation may be considered.
Use of mechanical chest compression devices
The use of mechanical chest compression devices has not shown improvement in the critical outcomes in OHCA and IHCA [84-90]. However, the use of these devices may be considered in specific settings where high quality manual compressions are not possible or may harm the providers (e.g., limited personnel, in a moving ambulance, during coronary angiography, during prolonged CPR, during eCPR, or risk of exposure to infectious disease such as COVID-19) [20,91].
eCPR
eCPR refers to venoarterial extracorporeal membrane oxygenation and cardiopulmonary bypass during the resuscitation of a cardiac arrest patient. Providers can consider applying eCPR to patients who have not achieved spontaneous circulation or have experienced repeated cardiac arrests despite receiving high quality CPR and patients who are expected to have a good neurological outcome. Highly trained medical staff and proper equipments and facilities are necessary for successful application of extracorporeal membrane oxygenation [92,93]. eCPR is recommended in patients aged <75 years and those with an initially shockable rhythm, a cardiac etiology or reversible cause of cardiac arrest, a no-flow time of <5 minutes, an unsuccessful resuscitation despite receiving ALS for >10 minutes, and a total collapse time of <60 minutes [94-107].
ADDITIONAL CONSIDERATIONS DURING ALS
Proper oxygen and carbon dioxide targets during CPR and after ROSC
Based on previous CPR guidelines suggesting that a high oxygen level must be supplied during CPR and a study finding that the arterial partial pressure of oxygen was higher in resuscitated patients, it is recommended to supply 100% oxygen during CPR [108]. However, after ROSC, it is necessary to adjust the level of inhaled oxygen to maintain an oxygen saturation of 94% to 98% [109-111]. There is insufficient evidence for or against targeting mild hypercapnia compared with normocapnia in postcardiac arrest patients [109,110].
Factors for predicting outcomes during CPR
Since the ETCO2 level reflects the cardiac output generated by chest compressions, it could be used to predict the patient’s outcome during CPR. In intubated patients, ETCO2 levels of <10 mmHg after 20 minutes of ALS were associated with poor outcomes, which can be a factor for deciding on whether to terminate resuscitation [32,112]. However, this parameter should not be used alone as a prediction tool for terminating resuscitation during CPR and should not be applied to patients who have not undergone endotracheal intubation.
Application of ultrasonography during CPR
Experienced physicians can use an ultrasound, if it does not interfere with CPR, to diagnose reversible causes of cardiac arrest such as cardiac tamponade, pulmonary thromboembolism (PTE), and myocardial infarction as well as to identify the existence of cardiac motility in patients with a PEA rhythm [10,113-115]. Ultrasound can also be used as an alternative to ETCO2 capnography to confirm the correct placement of an endotracheal tube during CPR [116]. However, it should not be recommended as an independent predictor for terminating resuscitation.
PTE
Cardiac arrest due to PTE is the most dangerous complication of venous thromboembolism, and most cases of PTE are caused by deep vein thrombosis [117]. There is evidence of low certainty supporting the occurrence of intra-arrest thrombolysis in patients suspected with PTE [118-120]. There is also evidence of very low certainty supporting the performance of surgical embolization or percutaneous mechanical thrombectomy to resuscitate patients with cardiac arrest [121]. Therefore, thrombolysis is recommended in cardiac arrest patients suspected of PTE. Providers should also consider performing surgical embolectomy or percutaneous mechanical embolectomy in cardiac arrest patients diagnosed with PTE.
Cardiac arrest in pregnancy
In pregnant women over 20 weeks of gestation, the cardiac output might be decreased by 30% to 40% as a result of compression of the inferior vena cava and aorta by the uterus, which reduces venous return [122]. In this case, the effectiveness of chest compressions may be reduced. The easiest method to avoid compressing the inferior vena cava or aorta is by lateral uterine displacement, which can be achieved by placing the patient in a supine position on a firm surface and moving the uterus to the patient’s left by pushing it with one hand from the right side or pulling it with two hands from the left side [123,124].
When providing chest compressions, the provider may consider placing the chest compressions slightly higher than the center of the sternum, which is the standard position, to take account of the rise of the abdominal cavity and diaphragm during pregnancy [125,126]. However, there is still insufficient evidence about the proper positioning of chest compressions in pregnancy. Since there is no change in transthoracic impedance during pregnancy and there are no reports that defibrillation affects the fetal heart, defibrillation can be performed according to standard CPR guidelines.
If cardiac arrest is imminent or occurs in pregnant women, hysterotomy or cesarean section should be performed as soon as possible. Early delivery can improve the resuscitation outcomes by relieving compression of the aorta and vena cava [127]. Several studies of pregnant women with cardiac arrest showed that fetal survival rates were high when the cesarean section was performed successfully within 5 minutes of cardiac arrest [128]. Therefore, it is recommended to perform a cesarean section within 5 minutes when spontaneous circulation is not achieved even after performing CPR for the first 4 minutes in pregnant women who are at >20 weeks of gestation or whose uterus is palpable above the umbilicus.
RRT and early warning score in IHCA
“Early recognition and CPR team activation” constitute the first chain of survival from IHCA according to the 2000 Korean CPR guidelines. For successful resuscitation of patients with IHCA, an appropriate survival environment that includes operation of an RRT, training in ALS, establishment of a cardiac arrest treatment system, and quality improvement activities must be established [129-131].
An RRT is designed to detect patients who are deteriorating rapidly at an early stage and to respond appropriately [13]. The role of an RRT includes making decisions about transferring the patient to the intensive care unit, implementing a do-not-resuscitate order, performing intensive care including CPR, and planning care for a patient with terminal illness.
Most cases of IHCA exhibit a non-shockable rhythm at an early stage, which is generally preceded by respiratory failure or shock [81,132,133]. Therefore, the use of an early warning score can help the RRT detect a rapidly deteriorating patient. Hospital systems may use different early warning scores, but the NEWS (National Early Warning Score) and MEWS (Modified Early Warning Score) are well known [17].
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant (2020E330300) of the Korean Disease Control and Prevention Agency funded by the Ministry of Health and Welfare, Republic of Korea.
We thank Ms. So Yeong Kim (EMT) for her assistance with administrative affairs and Mr. Myung Ha Kim for his assistance with literature searches for updating Korean Guidelines for Cardiopulmonary Resuscitation. We also thank the Korean Association of Cardiopulmonary Resuscitation (KACPR) for supporting the process of proofreading.
