Prognostic value of the myeloperoxidase index for early prediction of neurologic outcome in acute carbon monoxide poisoning
Article information
Abstract
Objective
Carbon monoxide (CO) activates intravascular neutrophils through platelet-neutrophil aggregates, which cause neutrophil degranulation. This process causes the release of myeloperoxidase (MPO), proteases, and reactive oxygen species. The MPO index (MPXI) is a newly reported inflammatory marker that reflects the MPO level within neutrophils. The MPXI in conditions associated with neutrophil activation depends on the net effect of azurophil degranulation. This study aimed to determine whether the MPXI can predict neurocognitive prognosis 1 month after acute CO poisoning.
Methods
We included patients aged ≥16 years with acute CO poisoning from a cohort at a single tertiary academic hospital in Wonju, Korea, between January 2010 and May 2021. Data from 699 patients were analyzed. The neurocognitive outcome was assessed using Global Deterioration Scale scores and classified as favorable (score, 1–3 points) or poor (score, 4–7 points). The MPXI was determined within 1 hour of arrival to the emergency department.
Results
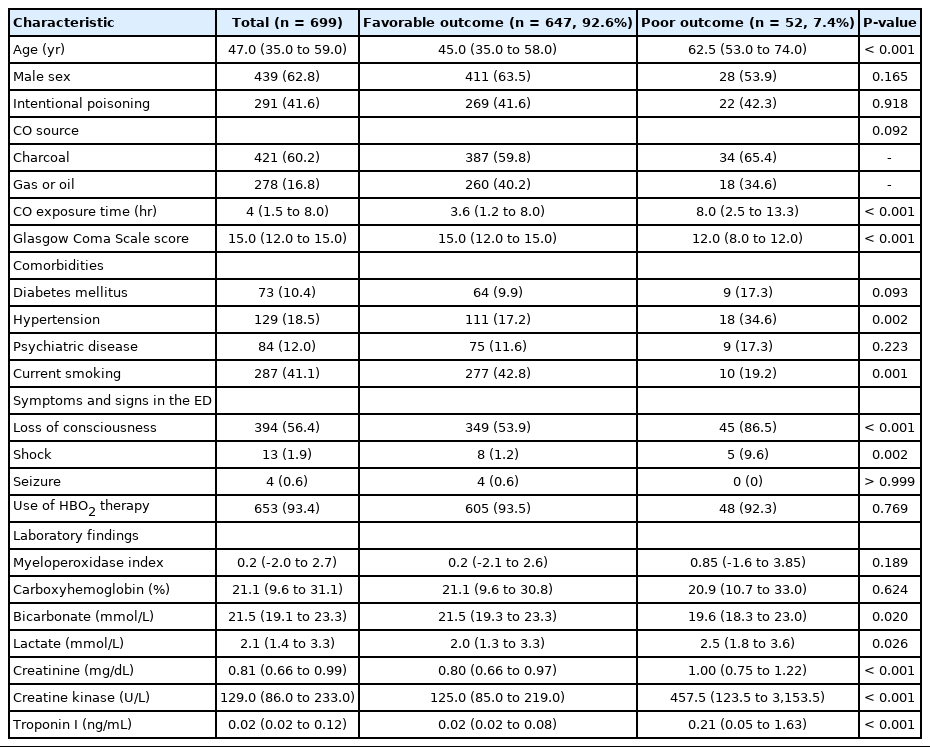
Among the 699 patients, 52 (7.4%) showed poor outcomes. The median MPXI of the patients in the poor outcome group was higher than that of the favorable outcome group (0.85 vs. 0.2, P=0.189). However, a significant difference was not found between the favorable and poor outcome groups, and MPXI was not a significant variable in multivariate logistic regression.
Conclusion
The MPXI evaluated in the emergency department did not differ based on neurocognitive outcome at 1 month after acute CO poisoning.
INTRODUCTION
Acute carbon monoxide (CO) poisoning can exhibit many symptoms and neurocognitive sequelae, including mental deterioration, cognitive dysfunction, amnesia, gait disturbance, mutism, urinary or fecal incontinence, psychosis, depression, and parkinsonism [1-3]. CO poisoning causes tissue hypoxia resulting from the high affinity of hemoglobin for CO and direct inflammatory damage to tissues through various mechanisms. CO competitively binds to heme-containing proteins (e.g., hemoglobin and myoglobin) and mitochondrial cytochrome c oxidase (complex IV), causing tissue hypoxia [4]. CO activates intravascular neutrophils through platelet-neutrophil aggregates, subsequently causing neutrophil degranulation [5]. This process causes the release of myeloperoxidase (MPO), proteases, and reactive oxygen species [5], leading to oxidative stress, transformation of xanthine dehydrogenase to xanthine oxidase in endothelial cells, lipid peroxidation, and apoptosis [5,6]. Furthermore, CO poisoning causes adduct formation between myelin basic protein and a reactive product of lipid peroxidation (i.e., malondialdehyde). Chemical modification of myelin basic protein is associated with an adaptive immunologic response that causes CO-mediated neurocognitive sequelae [7].
MPO plays an important role in this process. Exposure to CO triggers intravascular platelet-neutrophil interactions that lead to neutrophil degranulation, as observed in both experimental animals and human patients with acute CO poisoning [5]. In an animal model, CO poisoning reportedly increases the MPO level in the brain along the vascular lining and appears to cause vascular oxidative stress based on its colocalization with nitrotyrosine [5]. MPO can catalyze the reaction between nitrite and H2O2 to form nitrogen dioxide, which causes nitration of local protein tyrosine residues, induces lipid peroxidation, and stimulates expression of endothelial adhesion molecule [8-11]. MPO and nitrotyrosine have been shown to colocalize with each other along the subendothelial lining of human tissues of patients with inflammatory disease [12]. In a study by Thom et al. [5] platelet-neutrophil aggregates were detected in blood samples obtained from 50 consecutive patients, and the plasma MPO level was significantly elevated in patients with confirmed CO poisoning. Thom et al. [5] examined knockout mice lacking MPO and responses and showed a direct link between alterations in myelin basic protein and MPO. The MPO index (MPXI) is a newly reported marker for inflammation that reflects the MPO level within neutrophils [13]. In conditions associated with neutrophil activation, the MPXI might depend on the net effect of azurophil degranulation, which decreases the MPXI, and the stimulated synthesis of MPO in response to inflammation which increases the MPXI. Therefore, the MPXI can show specific patterns (attenuation, no change, or elevation) distinct from the plasma level of MPO or other biomarkers of inflammation, and further investigations on the association of MPXI with specific pathologic conditions might yield interesting findings [14].
Because the plasma MPO level is significantly elevated due to degranulation of neutrophils in patients with confirmed CO poisoning [5], we hypothesized the MPXI would be low in these patients. Therefore, we determined whether the MPXI can predict the neurocognitive prognosis of patients 1 month after CO poisoning.
METHODS
Ethics statement
The study was approved by the Institutional Review Board of Wonju Severance Christian Hospital (No. CR321138) and complied with the ethical guidelines of the Declaration of Helsinki. Informed consent was obtained and the patient data were anonymized before the analysis.
Study design and setting
The data used in this study were derived from a cohort at a single tertiary academic hospital in Wonju, Korea. The CO poisoning registry was established in January 2006 to prospectively collect data from consecutive patients at the hospital. Data from January 2010 to July 2020 were obtained from the existing prospective registry, and data from August 2020 to May 2021 were prospectively collected with informed consent (ClinicalTrials.gov identifier: NCT04490317).
The exclusion criteria were as follows: (1) age <16 years, (2) no acute CO poisoning, (3) history of a previous CO poisoning or a previous neurocognitive disease (e.g., stroke, dementia, or Parkinson disease) before acute CO poisoning, (4) any serious illness that could affect the outcome, (5) specific additive treatment such as therapeutic hypothermia or steroid, (6) cardiac arrest before emergency department (ED) arrival or at the ED, (7) failure to attend the follow-up examinations for neurocognitive status after discharge, (8) no available MPXI value determined within 24 hours after ED arrival, (9) hematologic disease and use of anticancer chemotherapy (which can influence the MPXI level or other important variables), (10) infections such as aspiration pneumonia diagnosed based on clinical symptoms, chest radiography findings, and sputum culture results (which can increase MPXI level), and (11) fire as the CO source.
At our institution, acute CO poisoning is diagnosed based on the patient’s medical history and a carboxyhemoglobin (COHb) level >5% (>10% for smokers). Patients with CO poisoning were treated with 100% oxygen therapy through a face mask with a reservoir bag. Patients with any loss of consciousness, any neurocognitive symptoms and signs, cardiovascular dysfunction, elevated levels of cardiac enzymes, ischemic electrocardiogram changes, severe acidosis, or COHb ≥25% were treated with hyperbaric oxygen (HBO2) therapy [15].
Study variables and definitions
Variables that can affect the prognosis of patients with acute CO poisoning were investigated, including age, sex, intentionality, CO sources, CO exposure time, initial Glasgow Coma Scale (GCS) score, comorbidities (diabetes mellitus, hypertension, and psychiatric diseases), current smoking, symptoms and signs (loss of consciousness, shock, and seizure), and use of HBO2. Shock was defined as need for a vasopressor and lactate level >2 mmol/L [16]. The investigated laboratory parameters were initial MPXI value and COHb, bicarbonate, lactate, creatinine, creatine kinase, and troponin I levels measured within 1 hour of ED arrival. At our institution, serum MPXI measurement is performed as a routine laboratory test using ADVIA 120/2120 (Siemens, Tarrytown, NY, USA).
The neurocognitive outcome was assessed using the Global Deterioration Scale (GDS) [17]. The scores GDS range from 1 to 7, and higher scores indicate a more severe condition. The GDS score was assessed during an outpatient rehabilitation visit. For patients in poor condition who could not attend outpatient rehabilitation, their caregivers were interviewed. The neurocognitive outcome was classified as favorable (GDS score, 1–3 points) or poor (GDS score, 4–7 points). If a patient died of CO poisoning (CO-related death) within 1 month, the outcome was expressed as a GDS score of 7. In addition, GDS was assessed at 6 months, and the changes between GDS values at 1 and 6 months were investigated.
Study outcome
The primary outcome in the present study was whether the MPXI measured at the ED was associated with the GDS score 1 month after CO poisoning.
Statistical analysis
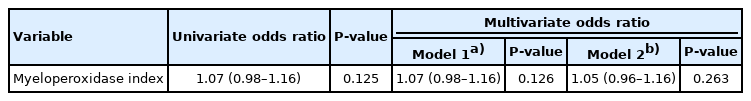
The normality of data distribution was determined using the Shapiro-Wilk normality test. Continuous variables with a normal distribution were expressed as mean (standard deviation) and compared using Student t-test. Continuous variables with a non-normal distribution were presented as median (interquartile range). Differences between the two groups were assessed using the independent t-test or Mann-Whitney U-test for continuous variables and the chi-square test or Fisher exact test for categorical variables. One-way analysis of variance was performed to compare the difference of MPXI based on time quartile from rescue to HBO2 therapy. Multivariable logistic regression was used to assess the independent association between MPXI and poor neurocognitive outcome after adjusting for continuous and categorical variables. Two adjusted models were constructed: model 1 was a minimally adjusted model, and model 2 included statistically significant baseline characteristics of age, CO exposure time, GCS, hypertension, smoking, loss of consciousness, shock, bicarbonate (mmol/L), lactate (mmol/L), creatinine (mg/dL), creatine kinase (U/L), and troponin I (ng/mL). All statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC, USA), and all graphics were produced using R ver. 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at P<0.05.
RESULTS
A total of 1,601 patients with acute CO poisoning visited the ED during the study period, and 699 were included in the present study (Fig. 1). Based on the 1-month GDS scores, the patients were divided into favorable and poor outcome groups. The favorable outcome group included 647 patients (92.6%) and the poor outcome group 52 patients (7.4%).
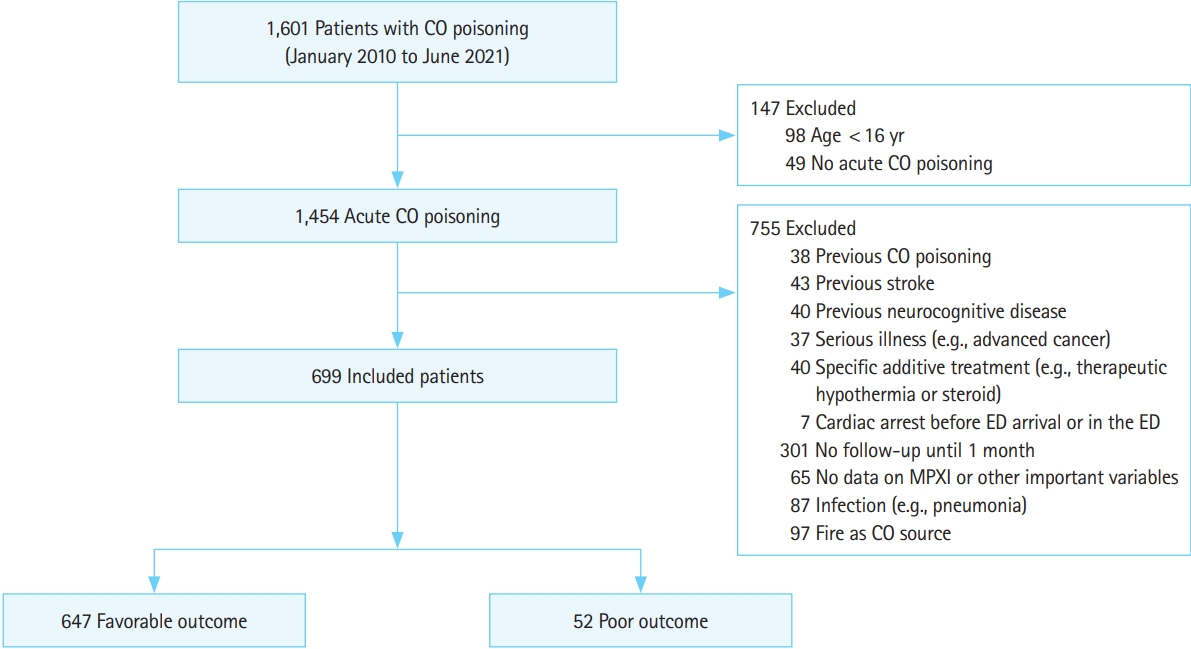
Flowchart of patient inclusion. CO, carbon monoxide; ED, emergency department; MPXI, myeloperoxidase index.
The baseline characteristics of the study patients are shown in Table 1. The median age was 47 years, and 439 patients (62.8%) were male patients. The CO originated from a nonfire source of charcoal (421 patients, 60.2%) and gas or oil combustion (278 patients, 16.8%), and 36.9% of the patients had intentional poisoning. Loss of consciousness occurred in 394 patients (56.4%), and 653 patients (93.4%) were treated with HBO2. The baseline characteristics of the patients divided based on the neurocognitive outcome are shown in Table 1. Patients in the poor outcome group were older than patients in the favorable outcome group (P<0.001). In addition, patients in the poor outcome group had longer CO exposure times (P<0.001), lower proportion of current smokers (P=0.001), and lower GCS score (P<0.001) compared with the favorable outcome group. Patients in the poor outcome group experienced hypertension, loss of consciousness, and shock more frequently (P<0.001) than patients in the favorable outcome group.
The results of laboratory tests are also shown in Table 1. Patients in the poor outcome group had higher levels of lactate, creatinine, creatine kinase, and troponin I than patients in the favorable outcome group. Serum bicarbonate level was higher in patients in the favorable outcome group than in the poor outcome group. MPXI level was not significantly different between the favorable and poor outcome groups. Contrary to our hypothesis, the MPXI level in the poor outcome group was higher than that of the favorable outcome group. Fig. 2 shows the MPXI values based on neurocognitive outcome. MPXI was not a significant variable in multivariate logistic regression (Table 2). In addition, when MPXI level was analyzed based on time from rescue to HBO2 therapy between favorable and poor outcome groups, significant difference was not observed (Fig. 3).

Comparison of myeloperoxidase index based on time from rescue to hyperbaric oxygen (HBO2) therapy between favorable and poor outcome groups. ANOVA, analysis of variance; Q, quartile.
The 6-month GDS score was obtained for 648 (92.7%) of the 699 patients: 586 subjects (90.4%) had no interval change in GDS score, 52 patients (8.0%) had a worse score, and 10 patients (1.6%) had improved GDS score (Table 3).
DISCUSSION
Because MPO is released from neutrophils in the initial inflammatory reaction process after CO poisoning, severe cases are expected to have higher MPO level. Therefore, we hypothesized the MPXI would be lower in the poor outcome group. However, the poor outcome group had higher MPXI levels than the favorable outcome group, contrary to our hypothesis. This finding can be attributed to several factors. First, the pathophysiology of acute CO poisoning includes hypoxia and inflammatory reactions such as platelet-neutrophil activation, oxidative stress, and cell apoptosis [2,4]. The increase in MPXI might have been due to inflammatory reactions. We postulate the MPXI would be lower if MPO was secreted from neutrophils through an inflammatory reaction. However, the change caused by the inflammatory reaction resulting from CO poisoning might have been stronger than expected leading to a further increase in the MPXI in the poor outcome group. Second, Thom et al. [5] and Thom et al. [18] reported the mean plasma MPO level to be five-fold higher in patients with CO poisoning (75.7 ng/mL) than in subjects without CO poisoning (control group, 15.0 ng/mL). However, the MPXI is analyzed differently from plasma MPO level. Plasma MPO level is directly measured in blood, whereas the MPXI reflects the MPO content in neutrophils and is a calculated, not a directly measured, value. The plasma MPO level increases in parallel with coronary atherosclerosis, and high plasma MPO level is associated with both severity of coronary lesions and prognosis of patients [19]. However, Yonezawa et al. [14] provided evidence that MPXI is not correlated with ischemic heart disease. They reported the MPXI is elevated in cases of milder arteriosclerosis obliterans but not in severe cases, and that the MPXI dramatically decreases when ischemic heart disease develops in patients with arteriosclerosis obliterans. Third, the MPXI might not be a sensitive tool because its value is derived through calculation, whereas the actual MPO level is directly measured in blood [14]. Cha et al. [20] reported the MPXI cannot differentiate sepsis from non-infectious systemic inflammatory response syndrome in patients diagnosed with systemic inflammatory response syndrome in the ED. Fourth, the time from the occurrence of CO poisoning to the blood test in the hospital differed among patients and might have affected the relationship between the MPO level released from neutrophils and the MPXI value in the present study. In addition, in patients with a relatively short CO exposure time, a significant MPXI decrease might not be reflected.
The present study had several limitations. First, this was an observational, nonrandomized study that involved only one hospital ED. Consequently, not all relevant parameters could be assessed. Second, several neurocognitive tests (approximately six), usually equivalent to CO batteries, were conducted in a few randomized controlled trials [21,22]. Conversely, in the present study, the outcome was only evaluated using the GDS score. In our institution, GDS scores are used to evaluate neurocognitive prognosis in patients with CO poisoning because neurocognitive functions, such as memory and concentration, as well as activities of daily living, are evaluated through interviews. We have previously reported the GDS score as a measure of neurocognitive outcome associated with CO poisoning [23-25]. Third, the MPXI was not continuously measured. The relationship between the MPXI and poor neurologic outcome over time after CO poisoning was not investigated. Fourth, accurately investigating the CO exposure time was sometimes challenging and especially difficult in unconscious patients. Because only patients with acute CO poisoning were included and subjects with chronic intermittent exposure were excluded, the CO exposure time was shorter than in a previous study [21]. Fifth, the predictive value of MPXI and other serum markers, including serum neuronal-specific enolase or S100β, was not compared [26,27]. Last, many patients were lost to follow-up due to their condition, a long distance from their residence to the hospital, or poor compliance.
In summary, the MPXI evaluated at the ED did not differ based on the neurocognitive outcome at 1 month after acute CO poisoning in the present study.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by the National Research Foundation (NRF) of Korea funded by the Korean government (the Ministry of Science and ICT) (No. NRF-2021R1A2C2004922). The authors have no relationships relevant to the contents of this paper.
References
Article information Continued
Notes
Capsule Summary
What is already known
Carbon monoxide (CO) causes the release of myeloperoxidase (MPO) and MPO plays an important role in CO toxicity. The MPO index (MPXI) is a newly reported inflammatory marker that reflects the MPO level within neutrophil. The MPXI in conditions associated with neutrophil activation may be dependent on the net effect of azurophil degranulation.
What is new in the current study
We aimed to determine whether the MPXI can predict the patients’ neurocognitive prognosis 1 month after CO poisoning. The MPXI evaluated in the emergency department did not differ according to the neurocognitive outcome at 1 month after acute CO poisoning.