Subdissociative-dose ketamine for sickle cell vaso-occlusive crisis: a narrative review for the emergency physician
Article information
Abstract
Vaso-occlusive crisis (VOC) in sickle cell disease can cause severe pain and requires a thoughtful approach to analgesia. Considering the heightened awareness of the harm associated with opioids, an exploration of safer and more effective alternatives is overdue. Ketamine may play a role in supplementing or replacing opioid analgesia for patients in VOC. Studies on the use of ketamine for VOC are sparse, though increasing. In this review, we summarize the literature on subdissociative ketamine use for VOC to offer providers insight into the most effective and safe dosing regimens for sickle cell disease patients. Overall, the studies discussed in this review show decreased opioid use when subdissociative ketamine is provided as an adjunct and resolution of pain for most patients when subdissociative ketamine is used as the sole form of analgesia in VOC. Most studies examined intravenous delivery, and successful outcomes were established for both adult and pediatric patients in multiple clinical settings, including in the emergency department. Although more research is needed, it is clear from the published data that subdissociative ketamine may provide an efficacious addition to the clinician’s toolbox for managing VOC.
INTRODUCTION
The social and economic burdens of sickle cell disease (SCD) cannot be understated: over 100,000 people in the United States live with this diagnosis, with annual costs of emergency treatment and hospitalization totaling over $1 billion [1]. Even though many of these patients have chronic pain due to this disease, 90% of SCD hospital admissions are due to acute vaso-occlusive crisis (VOC). VOC is defined as a new onset of pain that occurred within the 10 days prior to presentation, lasts at least 4 hours, and for which there is no explanation other than vaso-occlusion from a sudden increase in cell sickling [2]. VOC can be severe and requires a thoughtful approach to analgesia that minimizes morbidity from the methods of pain control selected. Typical treatment includes hydration and medications that fall under three major classes: opioids, nonopioids, and adjuvants. Opioids, such as morphine, hydromorphone, fentanyl, and tramadol, have traditionally been used [3]. They carry with them the risks of respiratory depression, overdose, and addiction, and the lesser-known risk of opioid-induced hyperalgesia, a phenomenon of increasing pain even in the setting of increasing doses of opioids. Opioid-induced hyperalgesia arises from overactivation of N-methyl-d-aspartate (NMDA) receptors, which leads to downregulation of opioid receptors. Clinically, this results in opioid-refractory pain, lengthy hospital stays, and high pain-related readmission rates [4]. Another factor that contributes to opioid-refractory pain in SCD is neuropathic pain, thought to occur from sensitization of spinal neurons with repeated vaso-occlusive events as SCD patients reach adulthood. The presence of a neuropathic process adds further complexity to the treatment of VOC, as opioids are often ineffective for neuropathic pain [5]. Innovative therapies are needed to more effectively treat pain in VOC.
Subdissociative (also known as subanesthetic, low, or analgesic dose) ketamine may provide an alternative or supplemental analgesic option by which to address pain in VOC. However, the US Food and Drug Administration currently only approves its use as an anesthetic agent, although some guidelines support the use of subdissociative doses of ketamine for pain control in children and adults [6]. Ketamine may be of particular use in SCD VOC as it is an NMDA receptor antagonist that prevents opioid tolerance and opioid-induced hyperalgesia; for example, ketamine improved the effectiveness of morphine in pain management [7]. In addition to the NMDA receptor antagonism of ketamine, it acts at cholinergic receptors, opioid receptors, and sodium and potassium channels as well, though these interactions are suspected to play ancillary roles in its analgesic effects [8]. Multiple studies have described the efficacy and safety of subdissociative-dose ketamine for analgesia in various settings, including the emergency department (ED) and inpatient settings [9]. However, studies on the use of ketamine in VOC are sparse though increasing [5,10-17]. There is variation among these studies in terms of ketamine dose, treatment duration, and concurrent opioid use.
In this review, we summarize the literature on subdissociative ketamine for VOC to offer both ED and inpatient providers insights into the most effective and safe dosing regimens for their patients.
METHODS
A National Center for Biotechnology Information (NCBI) Medline search included all articles published through March 2022, with the following terminology: “Anemia, Sickle Cell”[MeSH] AND “Ketamine”[MeSH]. Articles were excluded if they were classified as “review,” did not note the dosages of ketamine used, did not include key demographic details of the population studied such as age, were in languages other than English, or consisted of animal or cell culture experiments. We searched ClinicalTrials.gov for clinical trials using ketamine for VOC. Two reviewers (MHB and SMM) independently evaluated the eligibility of the references by reading their titles and abstracts. If there was any doubt about the relevance of the studies, we obtained full copies for further assessment. Data extraction was performed independently by both authors, and disagreements were resolved by consensus.
We focused on the use of ketamine for VOC and stratified our analysis into adult and pediatric patients (studies with subjects younger than 18 years or studies occurring in a children’s hospital setting). Within these two groups, we further substratified our analysis to examine efficacy and adverse effects based on method of medication delivery and dose.
RESULTS
Our search yielded 19 articles identified through NCBI Medline and 10 trials identified through ClinicalTrials.gov. Of the 19 articles identified through NCBI Medline, three were removed for being reviews, two were removed for being study protocols, one was removed for being unrelated to sickle cell patients, and five were removed for not having drug doses or primary outcomes listed. The remaining eight articles were included in this review. Of the 10 trials identified through ClinicalTrials.gov, two were completed with results available and were included in this review, while the others were either ongoing or discontinued due to poor recruitment.
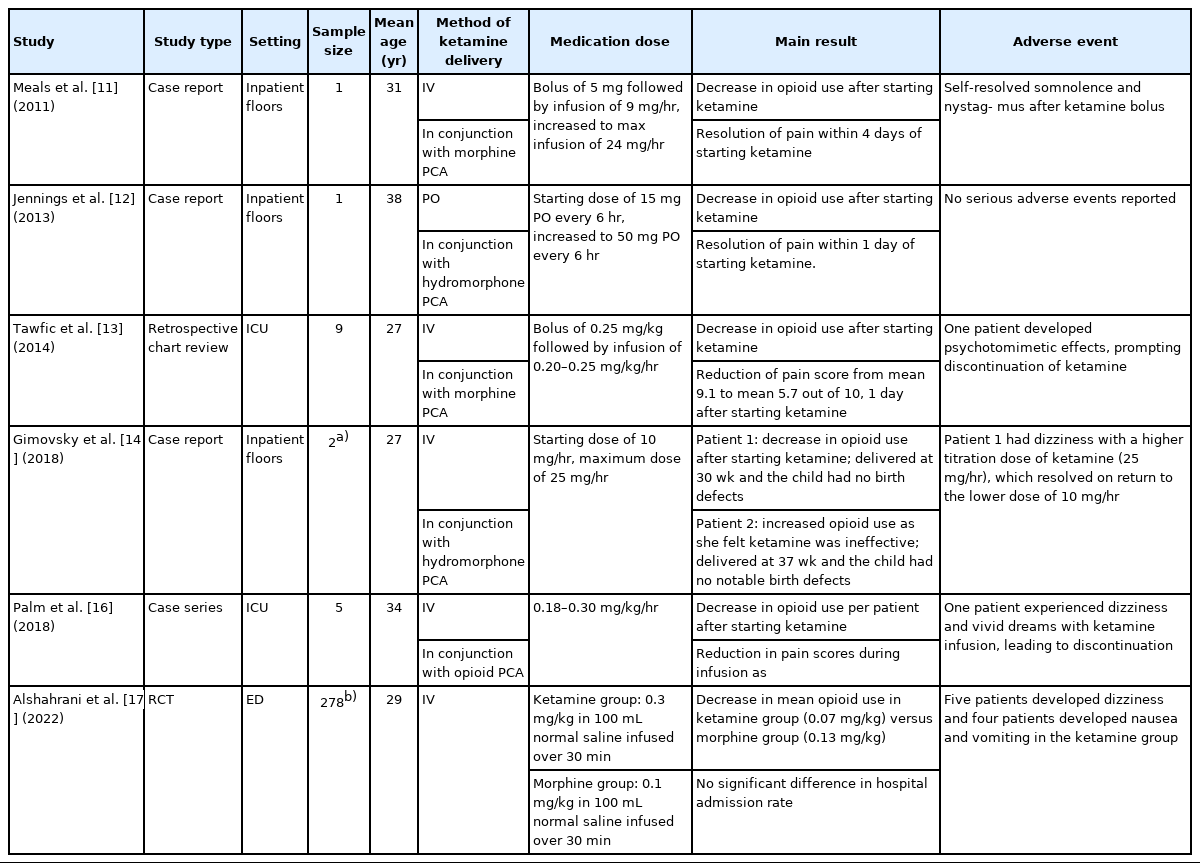
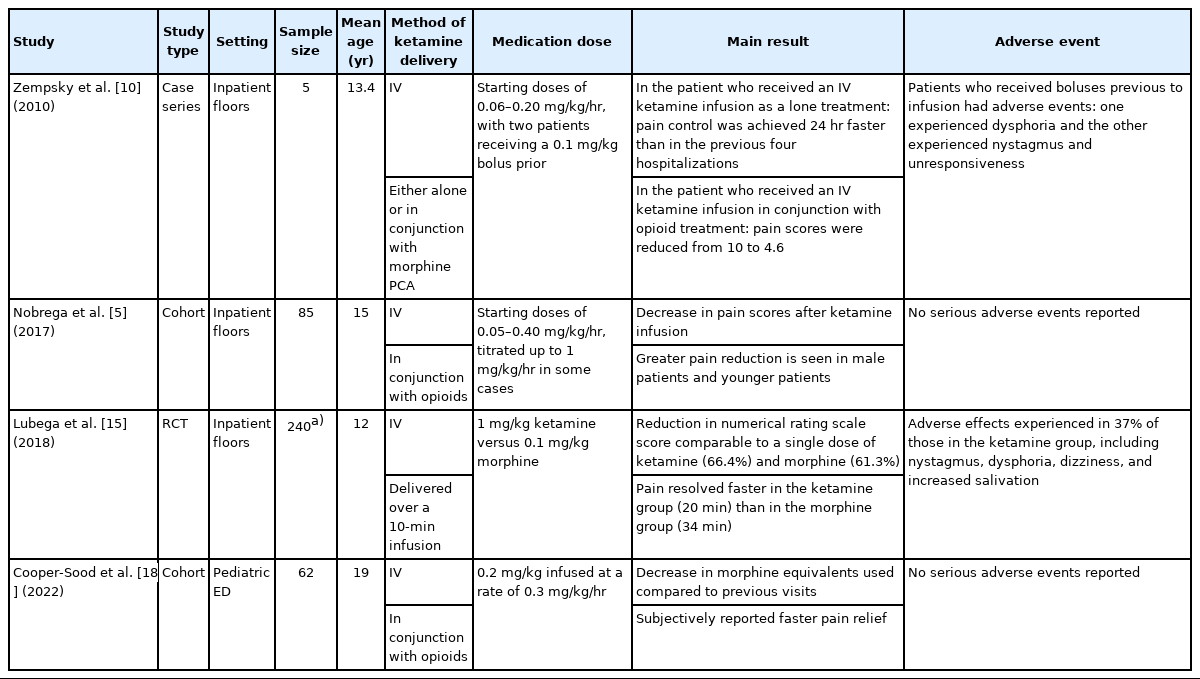
Of the studies identified, two were randomized controlled trials [15,17], two were cohort studies [5,18], and one was a retrospective chart review [13]. The remaining five studies were case reports [11,12,14] and case series [10,16]. Of the studies identified, two were conducted in the ED [17,18], six were conducted on general inpatient floors [5,10-12,14,15], and two were conducted in the intensive care unit (ICU) [13,16]. Six studies were stratified as adult studies (Table 1) [11-14,16,17] and four as pediatric studies (Table 2) [5,10,15,18].
Use of ketamine alone versus as an adjunct to opioids, method of ketamine delivery, use of a bolus versus infusion without a bolus, starting doses, and infusion times differed among the studies. Among the adult studies, five used ketamine in conjunction with opioids [11-14,16]. Opioid use was found to decrease after the initiation of ketamine in each of these studies. In one of these studies, ketamine was given as a 0.25 mg/kg bolus followed by an infusion of 0.20 to 0.25 mg/kg/hr for uncontrolled pain with increasing doses of intravenous (IV) morphine. In this study, a midazolam bolus of 1 mg followed by an infusion of 0.5 to 1.0 mg/hr was added to reduce the emergence of ketamine reactions. Mean IV morphine use decreased by 33 mg/day after ketamine was started (P=0.007), and pain scores, as measured by a numeric rating scale, decreased from a mean of 9.1 out of 10 before the initiation of ketamine to a mean of 5.7 out of 10, 1 day after ketamine was started (P=0.01) [13]. In another of these studies, a 10 mg/hr IV ketamine infusion was started in pregnant patients experiencing VOC unresolved with opioid therapy. In one of the two patients, ketamine treatment was effective in reducing concurrent IV hydromorphone use by 60% and resolving VOC, whereas it was not effective in the other. In both patients, deliveries occurred in the third trimester, and no notable birth defects were detected in either infant [14]. Another study in this group examined the use of oral (PO) ketamine in VOC with opioid therapy. Ketamine treatment in this study began with a starting dose of 15 mg every 6 hours, which was increased to 50 mg every 6 hours. IV morphine use was found to decrease by 1,000 mg daily after starting ketamine, and pain improved from activity-limiting to tolerable within 1 day of starting ketamine [12].
One study compared a group receiving a single low dose of ketamine (0.3 mg/kg) in 100 mL of normal saline or a standard dose of morphine (0.1 mg/kg) in 100 mL of normal saline relative to pain scores, hospital admission rates, and cumulative dose of opioids received. Though no significant difference was found in mean pain scores or hospitalization rates at the end of the trial, the ketamine group had lower mean opioid use than the morphine group [17].
Among the adult studies, two documented adverse effects led to treatment cessation in affected participants. In one of these studies, one patient (of nine) developed psychotomimetic symptoms after ketamine was first given as a bolus at 0.25 mg/kg and then infused at 0.25 mg/kg/hr [13]. In another study, one patient (of five) developed persistent dizziness and vivid dreams with a 0.18 mg/kg/hr ketamine infusion [16]. Other adverse effects included horizontal nystagmus that self-resolved [11], dizziness that resolved with a dose reduction [14], and nausea that was controlled with antiemetics [17].
Among the pediatric studies, one investigation examined both ketamine as a single treatment for VOC in one patient and ketamine in conjunction with opioids for others. In this study, one patient received only IV ketamine at 0.1 mg/kg/hr and had a hospital stay 24 hours shorter than a previous four hospitalizations for VOC, during which only opioids were received. Four patients in this study received IV ketamine with various dosing strategies after their pain was unresolved with opioids; the patient who received 0.1 mg/kg/hr without a bolus dose had a reduction in pain score from 10 to 4.6, whereas the patients who received boluses experienced adverse effects such as nausea and dizziness without relief of pain [10].
Two studies examined ketamine use in conjunction with opioids. In one of these studies, IV ketamine was initiated from 0.05 to 0.40 mg/kg/hr after unresponsiveness to opioid therapy. After initiation of ketamine infusion, patients had decreased pain intensity and opioid consumption (P=0.001). It was additionally found that younger age (P=0.018) and male sex (P=0.013) were predictors of greater pain reduction [5]. In another study, participants first received a dose of IV opioids followed by 0.2 mg/kg IV ketamine infused at a rate of 0.3 mg/kg/hr. Thereafter, pain scores, change in opioid usage compared to previous visits, and likelihood of discharge from the ED were assessed. It was found that patients subjectively reported faster pain relief with incorporation of ketamine into treatment and had decreased morphine equivalent usage compared to previous visits (P=0.004). The likelihood of discharge from the ED was not affected [18].
Last, a randomized clinical trial allocated 240 patients into two groups: one group received 1 mg/kg of IV ketamine, whereas the other received 0.1 mg/kg of IV morphine. Pain was assessed at regular intervals, and though no overall difference in pain scores was found at the end of the study, the ketamine group displayed a faster reduction in pain scores. The ketamine group also had a greater occurrence of adverse effects, with 37% experiencing nystagmus, dysphoria, dizziness, or increased salivation. Adverse effects were seen in 3% of the morphine group and included dizziness and urticaria [15].
DISCUSSION
Subdissociative use of ketamine has increased in EDs, inpatient floors, and ICUs for a growing number of indications [6]. Ketamine is affordable and accessible in resource-limited settings, can be delivered through various routes, and, as discussed in our paper, may play a role in both supplementing or replacing opioid analgesia in conditions such as SCD VOC. In light of the opioid abuse crisis and heightened awareness of the associated harms, including constipation, respiratory depression, dependence, and withdrawal, exploration of safer and more effective alternatives is overdue, especially for patients who are frequently in pain such as those with SCD [19].
The studies we discuss in this review suggest that the use of ketamine in VOC has many advantages. In most studies where it was used in conjunction with opioids, ketamine resulted in a decrease in opioid use. In most studies where it was used as the sole agent for analgesia, ketamine resolved pain for all but two patients who experienced adverse effects. The effectiveness of ketamine for VOC may have multiple sources: it has known beneficial mood effects that can be helpful in the SCD population, as many of these patients have a history of anxiety and depression [20]; it can prevent opioid-induced hyperalgesia through its antagonism of NMDA receptors [4]; and it has ancillary analgesic effects through its actions on cholinergic receptors and μ-opioid receptors [8].
Though the studies examining ketamine use for VOC offer different doses, methods of delivery, and concurrent opioid management strategies, the following can be considered to maximize efficacy and minimize adverse effects. First, ketamine can be offered in the ED, inpatient floors, and ICU settings. Ketamine can be given to both pediatric and adult patients. Second, subdissociative ketamine for acute pain should not be given to those with poorly controlled cardiovascular disease, including labile hypertension, psychiatric conditions involving psychosis such as schizophrenia, severe hepatic dysfunction such as cirrhosis, and allergy to ketamine [6]. Third, ketamine can be given in a variety of ways, most commonly IV as either a bolus dose followed by a continuous infusion or a continuous infusion without a bolus. Fourth, though a study in this review used subdissociative IV ketamine successfully for VOC in pregnant women, there are limited data for use in this population. Fifth, there are no data to suggest that subdissociative ketamine is unsafe in patients with renal dysfunction. Sixth, ketamine boluses can be associated with increased side effects. Psychosensory effects increase at bolus doses above 0.3 mg/kg. If used, boluses can be given at 0.1 to 0.3 mg/kg [21]. If used as a bolus, ketamine should be given over 10 to 15 minutes to reduce psychosensory adverse effects while maintaining analgesic efficacy [22]. Although a few studies used a ketamine bolus up to 1 mg/kg for pain control [15], this dose could be too large to be considered subdissociative and is better suited for ED procedures such as endotracheal intubation or joint reductions. Lastly, most data for ketamine use in VOC are from IV ketamine use, and ketamine should be infused by IV. Infusions can start as low as 0.1 mg/kg/hr and should not exceed 1.0 mg/kg/hr in non-ICU settings [23].
Though one study included in our review successfully used PO ketamine for VOC [12], the overall evidence and guidance for PO ketamine use in acute pain are limited. When taken PO, ketamine is approximately 20% bioavailable compared to 100% in IV administration [24]. No studies examined the use of intranasal ketamine for VOC, perhaps due to the numerous patient factors that can affect bioavailability in this route, which has been shown to range from 25% to 50% [25].
The overall safety profile of subdissociative ketamine is reassuring; large studies of continuous infusions for various pain conditions, including VOC, have shown no lasting psychotomimetic adverse effects, no hemodynamic changes requiring vasoactive agents, and no life-threatening adverse events [26]. In our review, the adverse effects seen were primarily associated with bolus dosing and infusions at higher doses [10,11,13,14,17]. While lower doses of subdissociative ketamine appear safe to use for VOC, data are limited regarding the effects of repeated exposure to ketamine. This is an area for further research, as SCD patients can present numerous times per month for treatment of VOC.
A continued examination of ketamine use for VOC is in line with guidelines that advocate a multimodal approach, defined as two or more medications that provide analgesia through different mechanisms, in SCD pain management [27]. The benefits of continuing to explore alternatives to opioids, such as ketamine, are decreased burden of opioid-related side effects and abuse on healthcare systems. We provide this review to summarize the investigations that have been conducted on ketamine as an alternative or adjunct to opioid therapy for VOC. While further trials on various populations including children, adults, pregnant patients, and those who present frequently for infusions need to be conducted to form stronger conclusions on subdissociative doses of ketamine for VOC, we offer general suggestions based on data from previous trials.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.