Effect of corticosteroid administration on cardiac arrest: a systematic review and network meta-analysis of the timing of administration
Article information
Abstract
Corticosteroids may have a beneficial effect on the outcome of cardiac arrest (CA); however, it is not known whether the timing of corticosteroid use affects the outcome. We performed a systematic review and network meta-analysis to compare the efficacy of corticosteroid administration according to the timing. A favorable final outcome, as the primary study outcome, was defined as a combination of survival with good neurologic outcome and survival for 1 year. The secondary outcome was survival to discharge. Nine clinical studies were included. Corticosteroids administered during cardiopulmonary resuscitation (CPR; odds ratio [OR], 1.29; 95% confidence interval [CI], 1.11–1.51) and post-CA (OR, 1.47; 95% CI, 1.30–1.66) had a positive effect on the favorable final outcome compared to the control protocol (no corticosteroid administration), while those used prior to CA had a negative effect. Corticosteroids administered post-CA had a positive effect on survival to discharge compared to the control protocol (OR, 1.82; 95% CI, 1.02–3.27), while those used prior to CA and during CPR had no significant effect. Post-CA was evaluated to be the best administration timing for both outcomes. In conclusion, the timing of corticosteroid administration may be an important factor for the prognosis of CA. Corticosteroids administration post-CA and during CPR may have beneficial effects on CA outcomes.
INTRODUCTION
Cardiac arrest (CA) is referred to as the abrupt loss of heart mechanical activity, as confirmed by the absence of signs of circulation. Out-of-hospital CA and in-hospital CA are important causes of mortality and morbidity worldwide, and it is estimated that 360,000 and 290,000 cases occur annually in the United States, respectively, with only 10% to 12% and 25% to 40%, respectively, of patients surviving [1,2].
Currently, epinephrine is considered the only essential pharmaceutical therapy except for antiarrhythmic drugs in CA according to the current advanced cardiac life support (ACLS) guidelines [3,4]. Several studies have previously shown that relative adrenal insufficiency is common in patients after CA, and higher cortisol levels are associated with lower mortality from circulatory shock in animal models and humans [5-8]. These results suggest that the use of corticosteroids after CA can suppress inflammatory reactions, regulate catecholamine synthesis, and protect against ischemia-reperfusion injury [9]. Based on this concept, there have been various clinical studies that assess the effect of corticosteroid administration on CA, which showed inconsistent results [10-18]. Moreover, even the results of recent meta-analyses that included those clinical studies were inconsistent [19-22]. The designs of the clinical studies mentioned above are quite different, especially in terms of the timing of corticosteroid administration; in some cases, corticosteroids were administered during cardiopulmonary resuscitation (CPR) or after the return of spontaneous circulation (ROSC), while other patients had a history of recent corticosteroid use prior to CA. We hypothesized that this difference in timing caused the discrepancies observed in the results of existing clinical studies and meta-analyses.
We aimed to assess the effect of corticosteroid administration on CA and whether the timing of administration affects the outcome. Hence, we performed a network meta-analysis of clinical studies that assessed the effect of corticosteroid administration on CA with a head-to-head comparison of the outcomes according to the timing of corticosteroid administration and prior corticosteroid use.
METHODS
Protocol
This study followed the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) statement extension for network meta-analysis guidelines [23,24] and was prospectively registered in PROSPERO (international prospective register of systematic reviews; No. CRD42022331973) [25].
Search strategy and inclusion criteria
Two reviewers independently searched for studies published in English before May 2022 using PubMed, Embase, Scopus, Web of Science, and Cochrane Central Register of Controlled Trials. We combined the following search words: “cardiac arrest,” “heart arrest,” “cardiopulmonary arrest,” “cardiopulmonary resuscitation,” “ventricular fibrillation,” “CPR,” “advanced cardiovascular life support,” “ACLS,” “steroid,” “glucocorticoid,” “hydrocortisone,” “adrenal cortex hormones,” “methylprednisolone,” and “dexamethasone.” We also searched for unpublished trials and ongoing studies at ClinicalTrials.gov (The US National Institutes of Health Ongoing Trials Register).
The studies with the following characteristics were enrolled: (1) population: adults (≥18 years) with CA, regardless of the CA location, with an initial presenting rhythm of CA; (2) intervention: steroid use during and/or after CA; (3) control: no steroid use (i.e., placebo or conventional ACLS therapy); (4) outcome: survival to discharge and ROSC; and (5) design: randomized controlled trial (RCTs), case-control studies, and cohort studies. In addition, the intervention group of some studies was administered additional drugs, such as vasopressin.
We excluded animal studies, case reports or series without a control group, study protocols, commentaries, review articles, and irrelevant studies (i.e., insufficient information or not in the field of interest).
Data collection and quality (risk of bias) assessment
Two reviewers independently extracted data from a data-collection form, including study title, name of author(s), publication year, country, study design, patients and demographics, interventions, timing of corticosteroid administration, and outcomes.
Risk of bias was assessed by using the Revised Cochrane risk of bias tool (RoB 2) for RCTs and GRACE (Good Research for Comparative Effectiveness) ver. 5.1 checklist for observational studies [26,27].
Any discrepancies were resolved by discussion and consensus between two authors. In the case of a sustained disagreement, a third expert acted as a moderator.
Outcomes and intervention groups
Primary outcome was the favorable final outcome, which we defined as cerebral performance category 1 or 2 at the time of discharge or 1-year survival after CA. The secondary outcome was the survival to discharge. The timing of corticosteroid administration was categorized as administration prior to CA, during CPR, or post-CA. We classified all the cases in which corticosteroids were administered after ROSC into the post-CA group regardless of whether corticosteroids were administered during CPR or not. This was because our interest lay in whether corticosteroids were administered post-CA, when the patient is in a sepsis-like condition. Every “prior to CA” classification was based on the subject’s history before the CA event in the observational study, not the timing of the actual intervention in the trial.
Statistical analysis
We calculated and presented odds ratios (ORs) with 95% confidence intervals (CIs) for each outcome and each intervention. Only the number of subjects who achieved ROSC was used as the overall count of each intervention group for the studies that enrolled whole CA patients, in order to match the conditions with those enrolled only post-CA patients. A frequentist random-effects network meta-analysis was conducted using multivariate meta-analysis and meta-regression [28,29]. The design-by-treatment model was used to confirm the consistency of the entire network [30]. The node-splitting method was also used to check the inconsistency between direct and indirect estimates of the effect [31,32]. Ranking probabilities were estimated by the surface under the cumulative ranking curve for each outcome. We used STATA ver. 17 (Stata Corp., College Station, TX, USA) for statistical calculations.
RESULTS
Study search results and characteristics
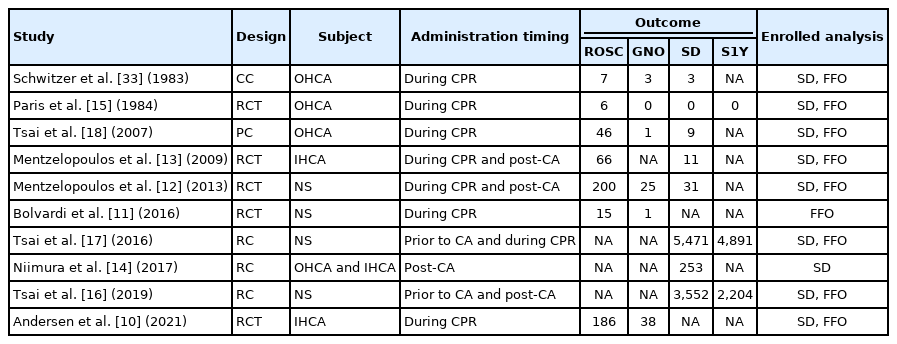
We identified 6,173 records through a database search and screened 5,018 after duplicates were removed (Fig. 1). Among these, 114 articles went through a full-text review, and 10 articles were finally selected by applying the inclusion criteria, including one casecontrol study [33], five RCTs [10-13,15], one prospective cohort study [18], and three retrospective cohort studies (Supplementary Table 1) [14,16,17]. Characteristics of the included studies are summarized in Table 1 [10-18,33].
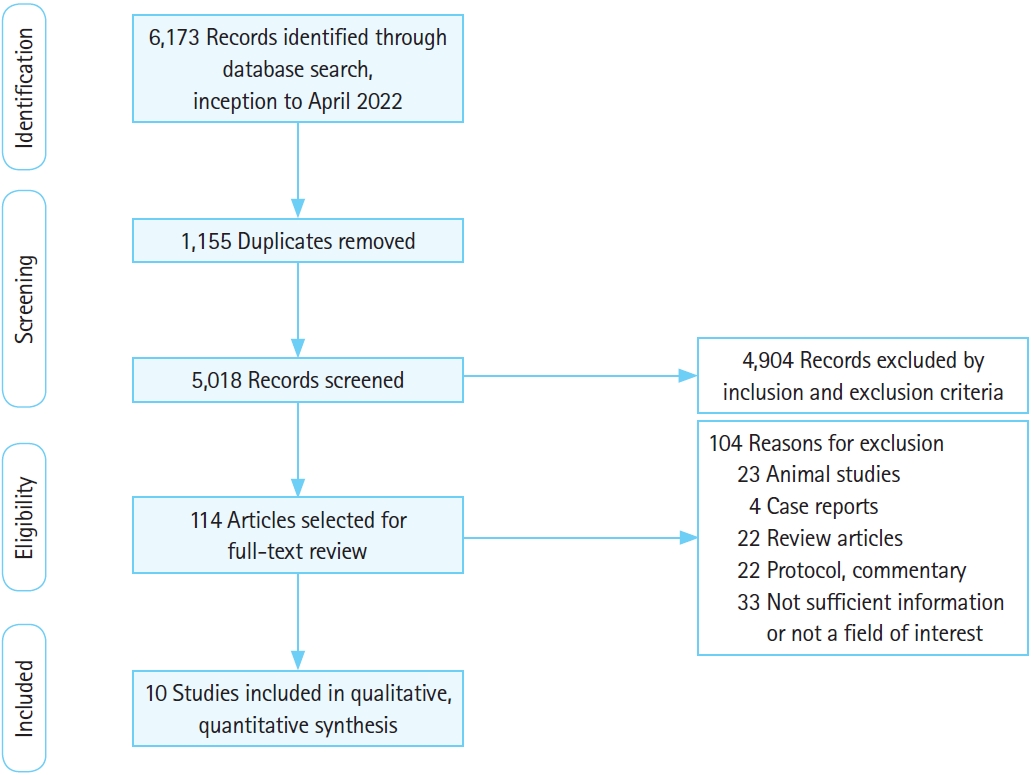
PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) flowchart and study selection.
Risk of bias in the enrolled studies
Most of the trials and all the cohort studies reviewed in the current meta-analysis were classified as those with good quality, according to RoB 2 assessment, except one trial with a high risk of bias (Supplementary Fig. 1). One case-control study was classified as a study with an uncertain risk of bias because of its lack of detailed information [33].
Favorable final outcome
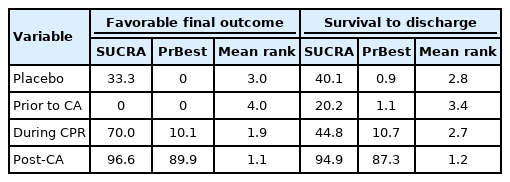
Nine studies were included for the analysis to determine the favorable final outcome [10-13,15-18,33]. The network plot of the analysis for the favorable final outcome is depicted in Fig. 2A. We did not find any inconsistency in the entire network (Supplementary Table 2). There were significant positive effects on the favorable final outcome associated with corticosteroids administered during CPR (OR, 1.29; 95% CI, 1.11–1.51) and administered post-CA (OR, 1.47; 95% CI, 1.30–1.66), compared to the control protocol (no corticosteroid administration), while there was a negative effect associated with their administration prior to CA (OR, 0.70; 95% CI, 0.64–0.77). Corticosteroids administered during CPR and postCA showed a significant positive effect on the favorable final outcome compared to that administered prior to CA (OR, 1.85; 95% CI, 1.56–2.18 and OR, 2.10; 95% CI, 1.85–2.39, respectively). Corticosteroids administration post-CA did not have a significant effect on the favorable final outcome compared to administration during CPR (OR, 1.14; 95% CI, 0.94–1.38) (Fig. 3) [10-13,15-18,33]. Post-CA was shown as the best timing for corticosteroid administration to achieve a favorable final outcome with a probability of 89.9% (Table 2).

Network maps of (A) the analysis for the favorable final outcome and (B) the survival to discharge. Network plot includes history of recent corticosteroid use prior to cardiac arrest (CA), corticosteroid administration during cardiopulmonary resuscitation (CPR), and corticosteroid administration post-CA.
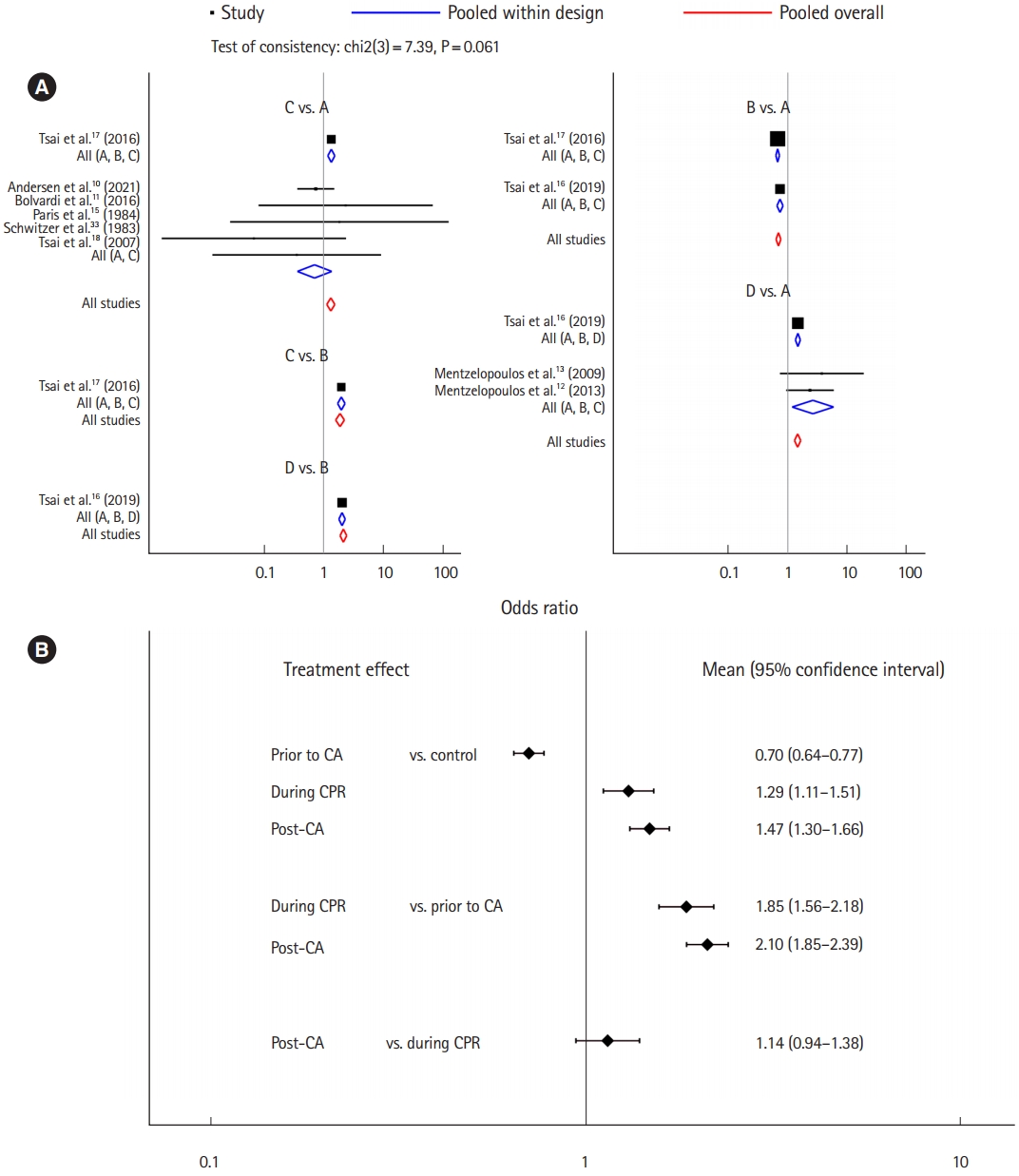
The analysis for the favorable final outcome. (A) Forest plot and (B) interval plot. In treatment arm: A, control; B, history of recent corticosteroid use prior to cardiac arrest (CA); C, corticosteroid administration during cardiopulmonary resuscitation (CPR); and D, corticosteroid administration post-CA.
Survival to discharge
Nine studies were included for the analysis of survival to discharge [10,12-18,33]. The network plot of the analysis for the survival to discharge is depicted in Fig. 2B. We found an inconsistency in the network (P<0.001), and the node-splitting method detected an inconsistency between corticosteroid administration prior to CA and during CPR (Supplementary Table 3). There was a significant positive effect on survival to discharge when corticosteroids were administered post-CA compared to the control protocol (OR, 1.82; 95% CI, 1.02–3.27), while there was no significant effect associated with administration prior to CA (OR, 0.83; 95% CI, 0.45–1.55) or during CPR (OR, 1.02; 95% CI, 0.51–2.05). Corticosteroid administration post-CA showed a significant positive effect on survival to discharge compared to administration prior to CA (OR, 2.19; 95% CI, 1.04–4.63), but no significant effect of administration during CPR existed (OR, 1.23; 95% CI, 0.55–2.72). Corticosteroid administration post-CA did not have a significant effect on survival to discharge compared to administration during CPR (OR, 1.79; 95% CI, 0.72–4.47) (Fig. 4) [10,12-16,18,33]. Post-CA was shown as the best timing for corticosteroid administration to achieve survival to discharge with a probability of 87.3% (Table 2).
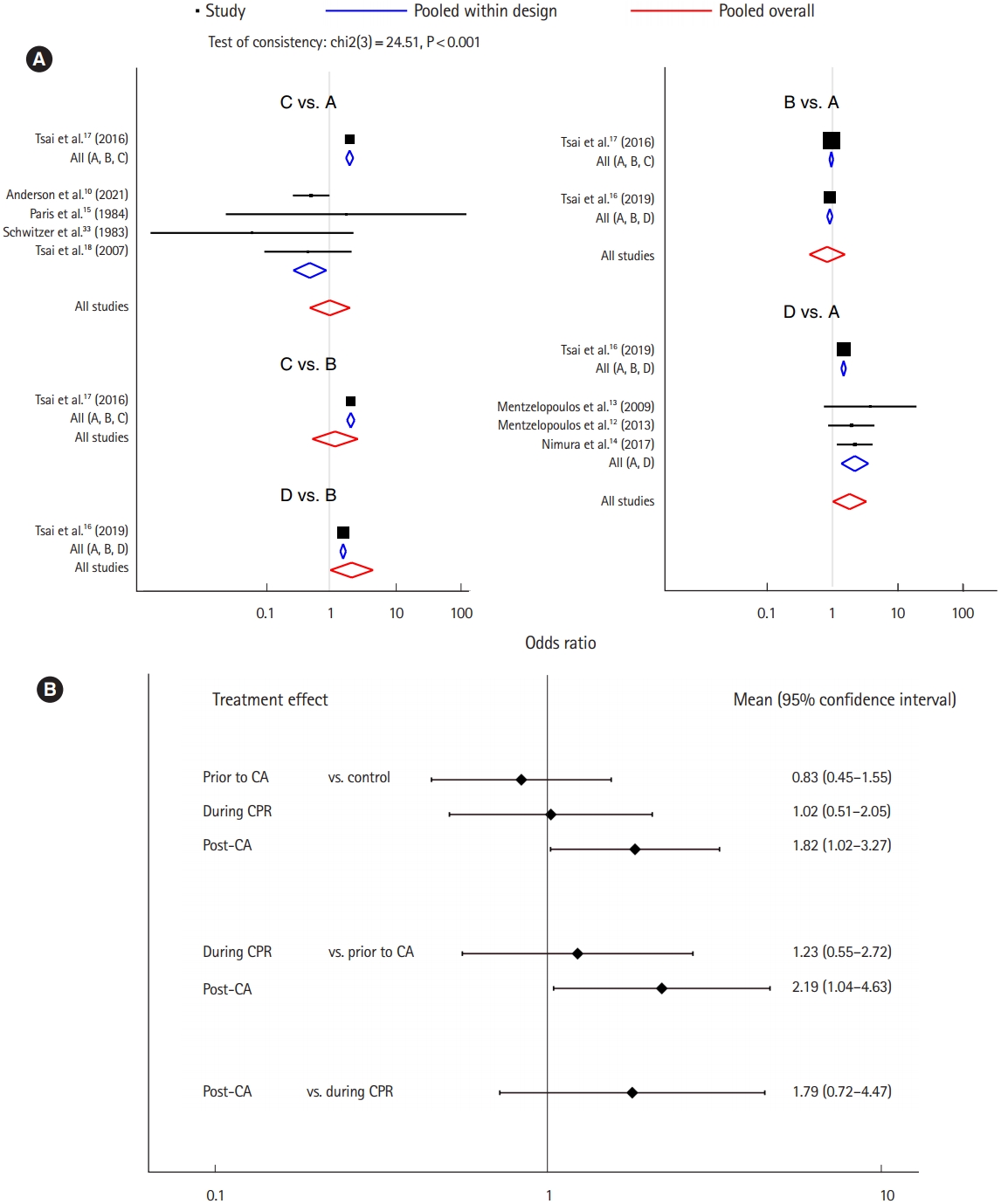
The analysis for survival to discharge. (A) Forest plot and (B) interval plot. In treatment arm: A, control; B, history of recent corticosteroid use prior to cardiac arrest (CA); C, corticosteroid administration during cardiopulmonary resuscitation (CPR); and D, corticosteroid administration post-CA.
DISCUSSION
To the best of our knowledge, this was the first network metaanalysis ever performed regarding the effect of corticosteroid administration on CA. The results of our current network meta-analysis suggest that the timing of corticosteroid administration may affect the outcome of CA patients. They also suggest that the discrepancy among the results of previous clinical studies assessing the effect of corticosteroid administration on CA may be due to differences in the study setting regarding the timing of administration. We can assume that the discrepancy among the metaanalyses that enrolled those studies could be due to differences in the setting of enrolled studies in each meta-analysis.
CA is a devastating medical condition that leads to high socioeconomic costs from which only a small portion of patients can achieve meaningful recovery. Its poor prognosis results from global cerebral ischemia-reperfusion injury, systemic inflammatory response, activation of the apoptotic pathway, free radical production, and myocardial injury [34-37]. It also causes disorders of the immune system and hemodynamic instability with increased levels of endotoxins and cytokines, i.e., a so-called sepsis-like syndrome [34]. One study showed that the serum cortisol level was decreased after ROSC and was associated with short-term survival [38]. These results initiated studies evaluating the effect of corticosteroid administration on CA. As a result, it was known that corticosteroid administration in CA improves cardiovascular stability through control of the systemic inflammatory response and improvement of the vascular response to vasopressors by lowering the catecholamine reuptake; it also helps to reduce myocardial and cerebral injury by reducing oxidative stress [39-43].
Many clinical studies that assessed the effect of corticosteroids on CA showed inconsistent results; therefore, the use of corticosteroids for the treatment of CA is recommended in a limited fashion with uncertain value in current guidelines for CPR [3]. Our current study suggests that the timing of administration may be an important factor in the effect of corticosteroids on CA. Based on this, further research directly comparing the effects on CA according to the timing of corticosteroid administration is necessary to establish a clinical guideline for corticosteroid use during CA treatment.
One of the most interesting findings in our research was that the outcomes of CA were significantly worse in patients to whom corticosteroids were administered prior to CA compared to controls; otherwise, the outcomes were significantly better than control group outcomes if corticosteroids were administered during CPR or post-CA. Considering that patients administered corticosteroids prior to CA, when included in our network meta-analysis, included those in whom a history of corticosteroid administration was identified during a short-term period before the CA event, it is likely that this group included chronic corticosteroid users. Adrenal insufficiency associated with chronic corticosteroid use may explain the worse prognosis in this group [44]. However, this result would better be accepted as a phenomenon showing that the prognosis is worse than that of the control group in a situation where relative adrenal insufficiency is suspected rather than as a recommendation for the timing of administration, considering that the “prior to CA” classification in original studies was not based on an actual timing of intervention but instead was a result of retrospective observation.
The results of our study also suggest that the effect of corticosteroids on CA may be better when they are administered after CA than when administered during CPR. We failed to identify a statistically significant difference in the indirect comparison of the effects of corticosteroids administered during CPR and those administered after CA, although there were consistent tendencies showing a relative superiority of their administration after CA. However, the results of the ranking probability tests for both outcomes support the better effect of corticosteroid administration after CA. Moreover, corticosteroid administration during CPR did not even show a significant difference in the comparison with the control protocol for survival to discharge. Considering the sepsis-like condition of post-CA patients, timely administration of corticosteroids targeting this condition may explain the better effect. Recent studies showing that corticosteroid administration after cerebral ischemia-reperfusion reduces neurological damage through the inhibition of apoptosis and inflammatory responses may also support this finding [45,46].
The results of the inconsistency test using the design-by-treatment model showed that the analysis of the primary outcome met the assumptions about consistency and homogeneity to some extent [28]. Therefore, it showed that our result from the analysis for the favorable final outcome could be reliable. On the other hand, the result of the analysis for survival to discharge, the secondary outcome, which did not meet those assumptions, may be difficult to accept. However, it could be adopted as a supportive result for the analysis of the favorable final outcome, considering that the results of both analyses showed a similar trend.
Our network meta-analysis had a few limitations. First, we used a newly defined outcome measure, the favorable final outcome, as a primary outcome, instead of a widely used one, such as survival with good neurologic recovery. This was because information regarding neurologic recovery was not available in two studies with the largest effect sizes [16,17]. Those studies used 1-year survival as an outcome measure reflecting long-term prognosis. Hence, we had to define a new outcome measure that can reflect the ultimate prognosis as much as possible, which was a combination of survival with good neurologic function and 1-year survival. Second, the design and quality of the studies included in our analysis vary compared to their number, and this may compromise the consistency and homogeneity, which are important for the reliability of a network meta-analysis. Hence, caution in adopting the results of our study despite our efforts to overcome this limitation, such as the use of design-by-treatment model or publication bias assessment, should be taken. Third, the analysis for the secondary outcome, survival to discharge, did not meet the consistency assumption of a network meta-analysis. Hence, one should be careful to adopt the result of the analysis for survival to discharge, especially for the comparison between corticosteroids administered prior to CA and those administered during CPR, which showed inconsistency. Fourth, we also classified studies with co-administered drugs such as vasopressin into the corticosteroid administration group, as in most of the prior pairwise meta-analyses dealing with the effect of corticosteroids on CA [19,21,22]; therefore, the possibility of errors due to differences in the intervention regimen cannot be excluded. Hence, caution is required in adopting our results because there might be an interaction of co-administered drugs that affects the efficacy of corticosteroids on CA. Fifth, our post-CA classification included cases of corticosteroid administration during CPR as well as after ROSC. Therefore, we could not rule out the possibility that our results, which showed that post-CA could be a better timing of corticosteroid administration, were attributable to the effect of repeated administration. Finally, our results did not contain enough direct comparisons for checking inconsistencies. Further research to directly compare the effects of corticosteroid administration according to the timing of administration is necessary.
In conclusion, the timing of corticosteroid administration may be an important factor for the prognosis of CA. Corticosteroids administration post-CA and during CPR may have beneficial effects on the outcomes of CA compared to the control protocol. Corticosteroids administration post-CA may have better efficacy than administration during CPR. Also, our data suggest that corticosteroid use prior to CA may be associated with poor outcomes.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at https://doi.org/10.15441/ceem.22.371.
Supplementary Table 1.
Search strategy
Supplementary Table 2.
Pairwise and loop inconsistency estimates of the analysis for the favorable final outcome
Supplementary Table 3.
Pairwise and loop inconsistency estimates of the analysis for the survival to discharge
Supplementary Fig. 1.
Risk of bias assessments.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: TNC; Data curation: all authors; Formal analysis: all authors; Investigation: all authors; Methodology: TNC; Project administration: TNC; Resources: TNC; Software: TNC; Supervision: TNC; Validation: TNC; Visualization: all authors; Writing–original draft: all authors; Writing–review & editing: TNC.
All authors read and approved the final manuscript.
References
Article information Continued
Notes
Capsule Summary
What is already known
Use of corticosteroids for the treatment of cardiac arrest may be beneficial in terms of return of spontaneous circulation, survival with good neurologic outcome, and long-term survival. However, the optimal timing for corticosteroid administration is unknown.
What is new in the current study
The timing of administration may affect the effect of corticosteroids on the prognosis of patients with cardiac arrest. Corticosteroids may be best administered after the return of spontaneous circulation regardless of whether they were administered during cardiopulmonary resuscitation or not.