The impact of the COVID-19 pandemic on in-hospital mortality in patients admitted through the emergency department
Article information
Abstract
Objective
The COVID-19 pandemic might have adversely affected outcomes of patients in emergency departments (EDs). The aim of this study is to evaluate the impact of the COVID-19 pandemic on in patients admitted through the emergency department.
Methods
This study is a single-center, retrospective, observational cohort study. We compared the prognosis of patients admitted through the ED before the COVID-19 pandemic (November 2018 to June 2019) and after COVID-19 (November 2020 to June 2021). The primary outcome was in-hospital mortality. Multivariable logistic regression analysis was performed to determine whether the COVID-19 pandemic was independently associated with patient prognosis.
Results
The number of patients admitted through the ED before and after COVID-19 was 5,333 and 4,625, respectively. The mean ED length of stay before and after COVID-19 was 401 and 442 minutes, respectively (P<0.001). The number of in-hospital deaths before and after COVID-19 were 269 (5.0%) and 322 (7.0%), respectively (P<0.001). Multivariable logistic regression analysis showed that the COVID-19 period was significantly associated with higher in-hospital mortality (adjusted odds ratio, 1.37; 95% confidence interval, 1.12–1.67; P=0.002).
Conclusion
In the COVID-19 period, in-hospital mortality increased compared to that before COVID-19 among hospitalized ED patients.
INTRODUCTION
The COVID-19 pandemic brought many changes to the medical environment and processes of emergency departments (EDs). The total number of patients visiting the ED decreased significantly during the COVID-19 period, although the proportion of admitted patients increased in one study [1]. The COVID-19 pandemic increased the number of people leaving emergency rooms without being seen [2,3]. One study found that ED length of stay (LOS) was significantly longer during the COVID-19 period than before, despite a smaller volume of patients in the COVID-19 period [4]. The time to provide medical care to patients was delayed due to concerns about the spread of COVID-19 in hospitals [5]. Admission to most Korean hospitals was allowed only after confirming negative COVID-19 polymerase chain reaction (PCR) results and isolating accordingly. In one study, PCR-based testing on admission was an effective component of COVID-19 diagnosis and reduced risk of in-hospital transmission [6]. During the pandemic, there were many efforts to decrease the time to a COVID-19 test result [7,8]. Nevertheless, the ED LOS for patients admitted through the ED continued to increase [4].
A number of studies have shown that the longer the LOS in the ED, the worse the patient’s outcome, because longer ED LOS often involves delay of timely and appropriate interventions, which increases condition severity and risk for poor outcomes [9–13]. Such treatment delays might have contributed to the development of time-dependent complications and subsequently higher intensive care unit (ICU) or general ward (GW) mortality [14]. For situations requiring urgent treatment, admission delays may worsen patient prognosis [15,16].
We hypothesized that COVID-19 had negative effects on patient prognosis. The purpose of this study was to determine whether there was a difference in in-hospital mortality among patients admitted through the ED before and after the COVID-19 pandemic.
METHODS
Ethical statements
The Institutional Review Board of Hanyang University Seoul Hospital of Korea approved the study (No. 2021-09-018). The requirement for informed consent was waived due to the observational nature of this study.
Study design and population
This is a retrospective observational cohort study conducted from November 2018 to June 2019 and from November 2020 to June 2021. This is a before-and-after study to compare differences before and after the COVID-19 pandemic. The study included adults over 18 years of age who visited the ED of a university-affiliated hospital located in Seoul, Korea. Data from patients admitted to the GW or ICU through the ED were extracted through chart review. All patients in the COVID-19 era who were admitted through the ED underwent COVID-19 PCR testing and were isolated accordingly. When COVID-19-related symptoms or epidemiological suspicions were observed and hospitalization was judged to be necessary, a routine PCR test or rapid molecular test (Xpert Xpress SARS-CoV-2, Cepheid) was performed. Results were obtained after 8 or 2 hours for routine PCR and rapid PCR, respectively. Exclusion criteria were direct transfer to another hospital from the ED, invalid admission code data, and do-not-resuscitate (DNR) orders.
Definitions and outcomes
The time-before-COVID-19 group included patients admitted to the GW or ICU through the ED of our hospital between November 2018 to June 2019. The after-COVID-19 group included patients admitted to the GW or ICU through the ED between November 2020 to June 2021. From November 2020, COVID-19 PCR was performed on all patients admitted to the GW or ICU through the ED. Only when the PCR test was negative was a patient admitted to the GW or ICU. PCR-positive patients were admitted to the COVID-19 isolation ward. The primary outcome of the study was in-hospital mortality. Secondary outcomes were ED LOS, use of mechanical ventilation, vasopressor use, continuous renal replacement therapy (CRRT), and ICU admission.
Statistical analysis
The study data are reported as mean±standard deviation or median with interquartile range for continuous variables as appropriate. Student t-test or the Mann-Whitney U-test was used to compare continuous variables. The chi-square test and Fisher exact test were used to compare categorical variables, the results of which are reported as absolute or relative frequency. A logistic regression model was used to assess the independent association of after COVID-19 period on in-hospital mortality, with multivariable adjustment for confounding variables that were significant in univariable analyses. Variables yielding P-values <0.1 in univariable analysis were entered into a backward multivariable logistic regression analysis. A P-value of <0.05 was considered significant. All statistical analyses were performed using SPSS ver. 18 (SPSS Inc).
RESULTS
Participant characteristics
A total of 5,455 patients was screened by chart review from November 2018 to June 2019. Of these, 49 patients with invalid admission code data, 22 patients who were directly transferred from the ED to another hospital, and 51 patients with DNR orders were excluded. Finally, 5,333 patients were included in the before-COVID-19 group. Of the 4,761 patients who were screened by chart review from November 2020 to June 2021, 77 with invalid data, 19 directly transferred from the ED to another hospital, and 40 with DNRs were excluded. Finally, 4,625 patients were included in the after-COVID-19 group (Fig. 1).

Flow chart of study participants. ICU, intensive care unit; ED, emergency department; DNR, do not resuscitate.
The mean age of patients in the before-COVID-19 and afterCOVID-19 groups was 60.2 and 62.6 years, respectively (Table 1). The mean ED LOS in the after-COVID-19 group was significantly longer than in the before-COVID-19 group (442 minutes vs. 401 minutes, P<0.001). The number of patients who received mechanical ventilation was 355 (6.7%) before COVID-19 and 195 (4.2%) after COVID-19 (P<0.001). In-hospital mortality before and after COVID-19 was 269 (5.0%) and 322 (7.0%), respectively (P<0.001). The other baseline characteristics of the study population are summarized in Table 1. During the COVID-19 period, there were 65 COVID-19 cases (1.4% of all cases), of whom 14 died (4.3% of all deaths). There were 2,284 (42.8%) and 2,238 patients (48.4%) admitted to internal medicine before and after COVID-19, respectively (Supplementary Table 1). The number of patients admitted to general surgery before and after COVID-19 was 425% and 433%, respectively.
Multivariable logistic regression analysis to predict outcomes
To examine the effect of the COVID-19 pandemic on patient in-hospital mortality, multivariable logistic regression was performed (Tables 2, 3). The COVID-19 pandemic was significantly associated with higher in-hospital mortality (adjusted odds ratio [aOR], 1.37; 95% confidence interval [CI], 1.12–1.67; P=0.002). Old age, heart rate, total bilirubin, creatinine, and lactate level were also independently associated with higher in-hospital mortality (aOR, 1.03, 1.01, 1.06, 1.08, and 1.14, respectively), as were systolic blood pressure and albumin (aOR, 0.99 and 0.35, respectively; both P<0.001). Additionally, we performed multivariable logistic regression analysis for factors predicting secondary outcomes. The COVID-19 pandemic was significantly associated with lower mechanical ventilator application, vasopressor use, and CRRT application (aOR, 0.45, 0.77, and 0.71, respectively; P<0.001, P=0.002, and P=0.031, respectively) (Supplementary Tables 2-4). However, the COVID-19 pandemic was not associated with increased ICU admission (aOR, 0.86; 95% CI, 0.74–1.01; P=0.06) (Supplementary Table 5).
Subgroup analysis
Patients transferred from external hospitals were excluded, and only patients who directly visited the ED were analyzed. In-hospital mortality in the after-COVID-19 group was significantly higher than in the before-COVID-19 group (6.7% vs. 4.5%, P<0.001) (Table 4). ED LOS of the direct ED-visited group was 413±423 minutes before COVID-19 and 467±406 minutes after COVID-19 (P<0.001). Significantly more patients presenting directly to our ED were treated with mechanical ventilation in the before-COVID-19 patient group than in the after-COVID-19 patient group (6.1% vs. 3.3%, P<0.001).
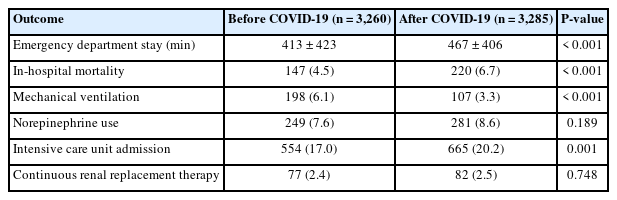
Comparison of outcomes before and after COVID-19 in patients presenting directly to emergency departments
We also compared outcomes of patients admitted to the ICU. Before COVID-19, 1,203 patients were admitted to the ICU from the ED; this number was 1,047 patients after COVID-19 (Table 5). The mean ED LOS was 425±451 minutes before COVID-19 and 451±460 minutes after COVID-19 (P=0.201). Mechanical ventilation was used significantly more often before COVID-19 than after (28.3% vs. 16.5%, P<0.001). In-hospital deaths before and after COVID-19 were 187 (15.5%) and 188 (18.0%), respectively (P=0.14).
DISCUSSION
After adjusting for confounding variables, in-hospital mortality of patients admitted through the ED in the after-COVID-19 period was significantly higher than before the COVID-19 period. Although we could not compare severity between the two groups, there were no differences in the proportion of vasopressor or CRRT use before and after COVID-19. Rather, the proportion of patients receiving mechanical ventilation was significantly lower after COVID-19 than before, although in-hospital mortality was higher. The number of patients admitted to the ICU from the ED did not differ significantly before and after COVID-19.
We investigated changes between the before-COVID-19 and after-COVID-19 periods for ED LOS and prognosis of patients admitted through the ED. Several studies have reported an association between ED LOS and patient prognosis. Furthermore, associations between COVID-19 and ED LOS have been reported [1,4,17–19]. The sample size of this study is relatively large. Another strength of our study is that the treatment protocol was consistent for all patients given the single-center study design. Although severity could not be directly compared between the time points, the variables that could affect patient prognosis did not show a statistical difference between the two periods, and some variables with a difference did not appear to have a clinical effect.
There are several possible explanations for a patient’s poor prognosis during the after-COVID-19 period. Since the spread of COVID-19, more people are staying at home to maintain social distance. Meanwhile, the numbers of remote treatments and postponed scheduled treatments are increasing [20]. In addition to the shift toward remote treatment, COVID-19 has also impacted hospital admissions and visits unrelated to COVID-19 itself [21]. Studies in Spain and Italy have shown a reduction in hospitalizations and procedures related to myocardial infarction and acute coronary syndrome [22,23]. Thus, it is possible that these patient did not receive timely treatment before their conditions worsened, and the severity was already high when patients arrived at the ED. In addition, as shown in an out-of-hospital cardiac arrest study, the longer the prehospital transport time, the worse the patient’s treatment outcome [24]. In patients who visited the ED for other conditions, the delay in prehospital transport due to lack of isolation rooms might be the cause of increased in-hospital mortality. However, our data do not have information about prehospital transport time and cannot be verified. Changes in the number and proportion of patients by hospital department before and after COVID-19 may also be associated with an increase in in-hospital mortality after COVID-19. Compared with the before-COVID-19 period, the number and proportion of internal medicine and general surgery inpatients increased after the COVID-19 pandemic. However, it is difficult to conclude that the increase in the number of patients admitted to internal medicine and general surgery in the after-COVID-19 period is the cause of the increase in in-hospital mortality without adjustment for severity or diagnosis. Increased workload, lack of rest, and fear of infection or infecting others were observed in the after-COVID-19 period, especially among healthcare workers in the ED and ICU [25]. These factors might adversely affect in-hospital patient care in the after-COVID-19 period.
Several studies have shown that ED crowding and long ED LOS are associated with poor patient prognosis, hospital LOS, and cost [10]. ED crowding increases the waiting time to enter the ED, which delays the final diagnostic test or definitive treatment. In our hospital, patients had to wait in the ED until their PCR test results were confirmed, and due to a lack of isolation beds, ED crowding increased. The wait time from ED arrival to meet with medical staff also seemed to increase, leading to an increased ED LOS. Similar to another study, the number of patients who left the ED without being seen was significantly higher after COVID-19 in our hospital [3]. A main reason for leaving without being seen was the long waiting time from arrival to ED entrance. In our hospital, people arrived at the ED but were often not registered immediately if there were many waiting patients. In these instances, although not specifically calculated, it is estimated that the actual ED LOS was longer in the period after COVID-19. Frequent shifts between nurses and doctors can impede continuity of care and prioritize new patients, resulting in poor-quality care. PCR testing is essential for COVID-19 diagnosis, and as the number of confirmed COVID-19 patients in Korea increased, most Korean hospitals were admitting patients to the GW or ICU after confirming their test results [26]. One study showed that the time spent in the ED increased after COVID-19 compared with before COVID-19 [27]. In that study, it was suggested that the cause of the increase in LOS and ED crowding was the time needed to obtain results from the COVID-19 PCR test. A study that analyzed changes in elderly patients before and after COVID-19 onset reported that the number of ED visits decreased after COVID-19, but that mortality increased, possibly due to the screening process or testing to differentiate between infected and noninfected patients [28].
This study has several limitations. First, because this is a single-center retrospective analysis, our results cannot be generalized to other medical institutions. Nevertheless, this single-center study has the advantage of a consistent treatment protocol, limiting the effect of treatment on patient outcomes. There is no international guideline stating that PCR results of all patients should be verified prior to admission from the ED to a ward or ICU. Therefore, it is not appropriate to generalize our findings regarding increased ED LOS to other medical institutions and other countries. Second, severity scores that could affect patient prognosis, such as sequential organ failure assessment score or acute physiology and chronic health evaluation scores, could not be analyzed. However, underlying disease, vital signs, and blood test results that might affect patient prognosis showed no clinically significant difference between the two groups. Moreover, proportions of vasopressor and CRRT use were not significantly different between the before-COVID-19 and after-COVID-19 periods. Considering that the frequency of mechanical ventilation has decreased since COVID-19, in-hospital mortality has not seemed to increase due to differences in severity. Finally, it cannot be concluded that an increase in ED LOS leads to an increase in in-hospital mortality. Variables that could affect a patient’s in-hospital mortality were adjusted through multivariable analysis; however, it is uncertain whether the adjustment is sufficient due to the possibility of unmeasured confounders. In addition, we cannot infer causality with our findings.
In conclusion, the after-COVID-19 period was significantly associated with increased in-hospital mortality among patients hospitalized via the ED. This study suggests that the outcome of ED patients might worsen during the COVID-19 pandemic, but further research is required about what factors affected the outcome.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at https://doi.org/10.15441/ceem.22.359.
Supplementary Table 1.
Comparison of the number of hospitalized patients by department before and after COVID-19
Supplementary Table 2.
Multivariable logistic regression analysis of mechanical ventilator use
Supplementary Table 3.
Multivariable logistic regression analysis of vasopressor use
Supplementary Table 4.
Multivariable logistic regression analysis of continuous renal replacement therapy
Supplementary Table 5.
Multivariable logistic regression analysis of intensive care unit admission
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article are reported.
FUNDING
This research was supported by the Bio and Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT of Korea (No. NRF-2019M3E5D1A01066060).
AUTHOR CONTRIBUTIONS
Conceptualization: THL, BSK; Data curation: CK, JL; Formal analysis: CK, JL; Funding acquisition: JO, HK; Investigation: JO, HK; Methodology: BSK; Project administration: BSK, CK; Resources: THL, CK; Software: THL, JO, HK; Supervision: JO, HK; Validation: JO, HK; Visualization: CK, JL, YC; Writing–original draft: CK, YC, JO, HK; Writing–review & editing: HK, THL, YC, BSK. All authors read and approved the final manuscript.
References
Article information Continued
Notes
Capsule Summary
What is already known
Numerous studies demonstrate the association between a prolonged stay in the emergency department and a poor outcome. However, there are few studies on the effect of COVID-19 on in-hospital mortality.
What is new in the current study
The COVID-19 period was significantly associated with increased in-hospital mortality among hospitalized patients via the emergency department. However, further research is required about what factors affected the outcome.




