Overview of clinical study designs
Article information
Abstract
The goal of a clinical study is to determine the factors associated with a disease and to assess the efficacy and safety of an investigational drug, procedure, or device. Since clinical study designs vary due to unique requirements of individual studies, the aims of this report are to educate researchers on the different types of studies and to assist researchers in choosing the optimal study type to fulfill their individual requirements. Clinical studies are classified into the two main types, observational studies and clinical trials, depending on the presence or absence of an intervention. Observational studies include case-control studies, cohort studies, and cross-sectional studies. Case-control and cohort studies may be prospective or retrospective, and case-control studies may be nested or not. Clinical trials may be pragmatic and may be controlled or noncontrolled; randomized or nonrandomized; open label or blinded; and parallel, crossover, or factorial. These observational and clinical trial designs are reviewed. Each type of clinical study has advantages and disadvantages. Therefore, researchers must consider these in choosing the design best suited for achieving their study objectives.
INTRODUCTION
Clinical studies are medical studies of groups of individuals. The goals of clinical studies are to determine associated disease factors and to assess the efficacy and safety of an investigational drug, procedure, or device for preventing, diagnosing, and treating disease. Clinical studies may test for the long-term effects or cost-effectiveness of an investigational treatment. There are two main types of clinical study, observational studies and clinical trials. In observational studies, investigators gather information on broad characteristics. For example, investigators may collect data through medical exams or questionnaires on the effects of lifestyle on cognitive health. Observational studies provide valuable information and may assist in identifying topics for clinical trials. Clinical trials test the safety and efficacy of medical, surgical, or behavioral interventions in individuals. Clinical study results have clinical, public, and economic impacts and need to be well-planned to provide valid study results.
Since study designs vary due to unique individual requirements, choosing the optimal study design is important. The aims of this article are to educate researchers on the different study designs and to assist those researchers in choosing optimal designs for fulfilling their research needs.
OBSERVATIONAL STUDY DESIGNS
Observational studies are those in which groups of individuals are monitored or outcomes are measured without manipulation or intervention to affect the result. Observational studies include case-control, cohort, and cross-sectional studies. Advantages and disadvantages of each type of observational study are listed in Table 1.
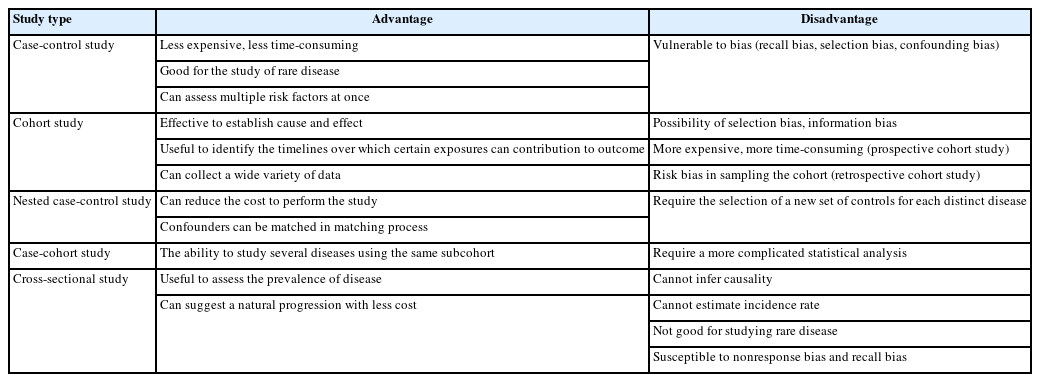
Advantages and disadvantages of a case-control study, cohort study, case-control study within a defined cohort, and cross-sectional study
Case-control study
Case-control studies compare groups, such as subjects with a disease or condition under study (cases) to subjects without the disease or condition (controls). Investigators study the medical or lifestyle histories of those in each group to determine factors that may be associated with the disease or condition (Fig. 1). If a factor is found more commonly in the cases than in the controls, the investigator may hypothesize that the exposure is linked to the disease. For example, in the investigation of risk factors for depression in intensive care unit (ICU) patients, the patients with depression were defined as cases; and sex, age, length of ICU stay, and individual medications were considered as risk factors associated with depression [1].
Advantages and disadvantages
The main advantages of a case-control study are low cost and low time consumption. The case-control approach allows for study of rare diseases that require lengthy study periods. The case-control study design allows assessment of multiple factors at once.
A disadvantage is that bias is inherent in case-control studies. Case-control studies have the potential for recall bias, the increased likelihood that those with the outcome will recall and report exposures more frequently than those without the outcome. Recall bias may lead to conclusion of false associations between exposure and disease.
One of the aspects that is often overlooked is the selection of cases and controls. Appropriate selection of cases and controls to obtain a meaningful and scientifically sound conclusion is important and can be achieved by matching. Matching assists in risk factor or etiological identification that cannot be explained by other differences between the groups. Thus, choosing a control group that bolsters the strength of the case-control study and enhances the researcher’s ability to find valid potential correlations between exposures and disease states is important.
In addition to bias, the investigator must recognize the potential for confounding factors that result when a variable that is not being accounted for is related to both the exposure and the outcome. The potential for confounding is another disadvantage of case-control studies.
Cohort study
Cohort studies are a type of longitudinal study, an approach that follows study participants over time. Specifically, cohort studies recruit and follow study participants who share common characteristics. Baseline information on the individual cohort members are gathered first to get detailed picture of the cohort. Then, investigators collect data from different time points in the study. Investigators compare the development of disease between two groups, the exposed and the nonexposed groups, generated from the baseline information. Also, by comparing data from the follow-up points, investigators can evaluate the effects of factors on health.
Prospective cohort study and retrospective cohort study
Cohort studies can be prospective or retrospective. A prospective cohort study investigates an event, for example, a disease, yet to occur in the group. A retrospective study investigates an event that has already occurred (Fig. 1).
Prospective cohort studies require recruitment of groups of participants to follow over time to gather new data. For exposuredisease correlations, investigators follow the participants from presence of exposure to development of disease. One prospective cohort study of syncope prognosis based on emergency department (ED) diagnosis generated a cohort from a group of adult patients with ED visits for syncope. The patients were followed for 30 days to investigate the frequency of serious outcomes and determine the factors associated with the outcome [2].
Retrospective cohort studies involve analysis of preexisting data. For exposure-disease investigations, retrospective cohort studies identify populations with and without an exposure based on past records and then assess disease development by the time of study. For example, to determine the effects of three aspects of care provided by primary physicians (physician specialty, continuity of care, and comprehensiveness of care) on patient use of the ED, investigators created a retrospective cohort of adults aged 18 years and older using provincial administrative databases that covered a 3-year span. The primary care variable and covariables were measured during an initial baseline period (the first 2 years of the study); visits to the ED for the primary outcome were measured during the last year of the study [3].
Advantages and disadvantages
One of the advantages of cohort studies is their effectiveness in establishing cause and effect. Cohorts are usually large, allowing investigators to draw relatively confident conclusions regarding the links between risk factors and disease. In many cases, because participants are often free of disease at the commencement of the study, cohort studies are particularly useful at identifying the timelines over which behaviors contribute to disease development. Another advantage is that investigators can collect a wide variety of data in cohort studies that can be used in multiple ways. A study on the impact of smoking, for example, might reveal links with multiple types of diseases. Investigators can also compare degrees of risk among risk factors.
Like case-control studies, cohort studies are subject to bias. Some of the biases observed with cohort studies include selection bias and information bias. Selection bias results when exposure is linked to study participation. Individuals with an exposure may refuse to participate in the study at a higher rate than those without the exposure. Selection bias also occurs when the exposed are lost to follow-up. Because of selection bias, interpretation of associations between exposures and outcomes is difficult. Information bias occurs when the data in past records are inaccurate in the evaluation of exposure status creating interpretation difficulties. Causal inference problems also result in prospective cohort studies when participants who are aware of their participation in a cohort alter their behavior during follow-up. In addition to bias, disadvantages of prospective cohort studies include their time consumption and expense compared to case-control studies. Retrospective cohort studies are more pragmatic; the use of historical data decreases time and expense requirements. However, retrospective approaches increase the risk of bias in sampling of the cohort due to missing data. Retrospective cohort studies are also weakened by the data fields available not being designed with the study in mind.
Case-control studies based within a defined cohort
This type of study combines some of the features of a cohort study with those of a case-control study design. When a defined cohort is embedded in a case-control study design, all baseline information is collected before the onset of disease, and the cohort is followed until onset of disease. One of the advantages of this design is the elimination of recall bias as the information regarding risk factors is collected before onset of disease. Case-control studies based within a defined cohort can be further classified into nested case-control studies and case-cohort studies.
Nested case-control study
This type of study design involves the selection of several controls for each case, typically from those still under observation at the time when the case developed the disease. A nested case-control study consists of a defined cohort with suspected risk factors and assignment of controls within the cohort to cases, subjects who develop the disease [4]. Over a period, cases and controls are identified and followed as per the study protocol. Hence, the case and control are matched in time and length of follow-up (Fig. 2A). When this study design is implemented, controls may become cases. The procedures for sampling in a nested case-control study follow. Select all those who become cases. Select controls randomly from those still at risk at time of case development (risk set), selection of five controls typically maximizes efficiency. Controls are time-matched to cases. Individuals can be controls more than once, and an individual selected as a control may later become a case. In the matching process, additional matching on confounders is often involved. One such study examined the association between incident injury after prescription opioid initiation and subsequent risk of opioid-related adverse events (ORAEs). The nested case-control study was conducted in a cohort of individuals 65 years and older. This assessment was of the association of prescription opioid use with recency of injury among older patients. ORAE cases were identified as patients who became inpatients or outpatients with a diagnosis code for opioid misuse, dependence, or poisoning. Using 1:4 matching, controls were randomly selected using incidence density sampling with matching criteria that included the year of cohort entry date and a disease risk score [5].
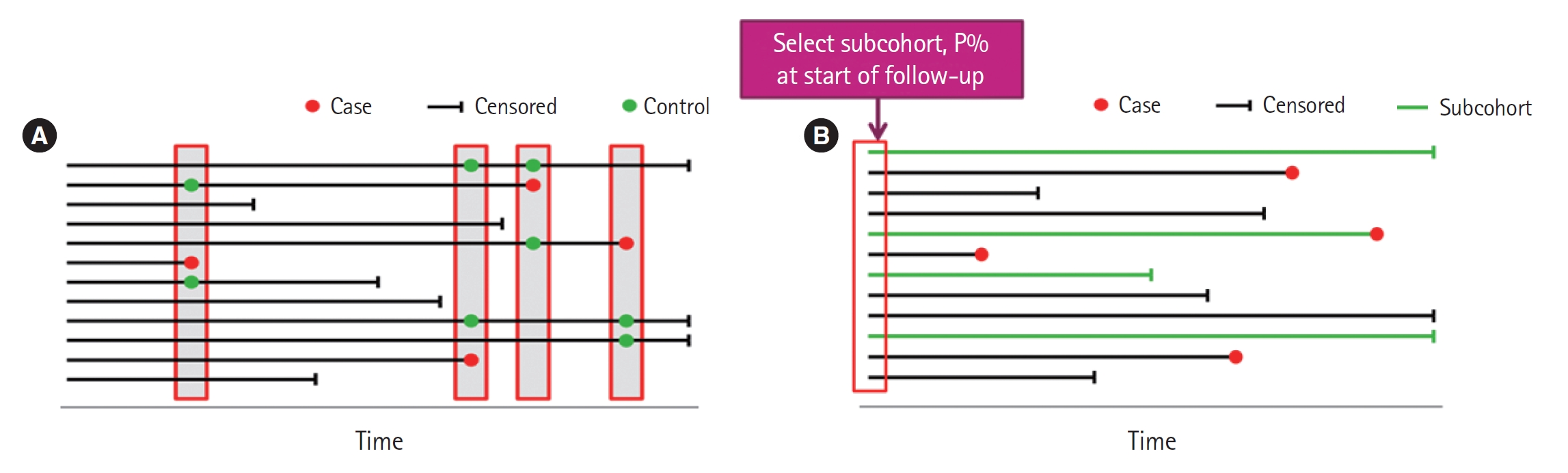
Case-control study based within a defined cohort. (A) Nested case-control study. Controls are time-matched to the cases. (B) Case-cohort study. Subcohort is not time-matched to the cases.
Nested case-control studies have some limitations. When more than one disease outcome is considered, a strict implementation of the nested case-control design requires selection of a new set of controls for each distinct disease outcome. Estimation of risk is not possible because the at-risk period is unknown. We can estimate rate, however, if the size and fraction of each risk set are known; but this is not a trivial matter, especially if there are time-dependent effects.
Case-cohort study
Case-cohort study designs were proposed as an alternative to the nested case-control study design. This design requires only selection of a subcohort random sample and all cases. Cases are defined as those participants of the cohort who developed the disease of interest, but controls are identified before the cases develop (Fig. 2B). Controls are randomly chosen from all cohort participants regardless of disease of interest status, allowing for early collection of baseline data. Case-cohort studies are similar to nested case-control studies; the main difference between the two study types is the way in which controls are chosen. A case-cohort study was conducted to examine the association between the risk factors and hospitalization in a cohort of dog bite victims requiring ED visits. The risk factors included infection, complicated injury, host defense abnormality, number of previous evaluations for the injury, and anatomic location of the bite. The case-cohort design was chosen because cases could be identified in a well-defined administrative cohort, medical record review was required for each study patient, and the risk ratio was the effect measure of interest. Cases were cohort members who were admitted as inpatients directly from the ED. From the cohort, a simple random sample was selected for the subcohort comparison group. Some patients were included into both subcohort and case groups [6].
Compared to the nested case-control studies, a major advantage of the case-cohort design is the ability to study several disease outcomes using the same subcohort. For example, investigators interested in determining if smoking is a risk factor for both diabetes and lung cancer would require two control groups with a nested case-control design, while a case-cohort design only requires one subcohort. Unlike the nested case-control study, the case-cohort study can estimate rate or risk, since the measurement in the subcohort can be observed for any time up to variable event onset.
A case-cohort study has some limitations. Information bias can be increased when the subcohort is established after baseline. With much censoring, the subcohort becomes “thin” and may not be representative of the cohort. Also, statistical analysis is more complicated than with a nested case-control study.
Cross-sectional study
A cross-sectional study is a type of observational study that involves data collected at a defined time; a cross-sectional study analyzes data from a population, or a representative subset, at a specific point in time. These studies are often used to assess the prevalence of acute or chronic conditions but cannot be used to answer questions about the causes of disease or the results of interventions. That is, cross-sectional data cannot be used to infer causality because temporality is not known. Cross-sectional studies may involve special data collection, including questions about the past, but often rely on data originally collected for other purposes.
Advantages and disadvantages
The use of routinely collected data allows large cross-sectional studies to be conducted at little or no expense. A natural progression has been suggested from cross-sectional studies of routinely collected data that suggest hypotheses, to case-control studies that test these hypotheses more specifically, to more costly and time-consuming cohort studies and trials that provide stronger evidence.
Temporal association cannot be established as the information is collected at the same time point. If a study involves a questionnaire, the investigator can ask questions about onset of symptoms or risk factors in relation to onset of disease. The prevalence of a disease can be determined; the incidence cannot. Cross-sectional studies are not suited for studying rare diseases and are susceptible to biases such as nonresponse bias and recall bias.
CLINICAL TRIAL
A clinical trial is a prospective study of the effects of interventions or manipulations of interest. Since this type of study can provide the most convincing demonstration of evidence of causality, the design requires meticulous planning and resources to provide an accurate result.
General considerations
When designing a clinical trial, selecting a representative population that assures generalizability to the target population is of paramount importance, as is selection of appropriate endpoints. Endpoints need to be well-defined, reproducible, clinically relevant, and achievable. The types of endpoints are continuous, ordinal, nominal, and time-to-event; and the endpoint is typically classified as primary, secondary, or tertiary. An ideal endpoint is a purely clinical outcome; for example, cure or survival, and clinical trials can be long and expensive. Surrogate endpoints may be biologically related to the ideal endpoint and need to be reproducible, easily measured, related to the clinical outcome, affected by treatment, and occur earlier than the clinical outcome.
Controlled vs. noncontrolled trials
Clinical trials are divided into controlled versus noncontrolled clinical trials depending on the presence or absence of a control group for the investigational treatment of interest.
Uncontrolled trials
Uncontrolled trials are often used in the early phases of drug research, phases I and II, to determine pharmacokinetic properties or to investigate tolerated dose ranges. Uncontrolled trials can also be useful to study side effects, biochemical changes in long-term therapies, tolerance, interaction, or efficacy of drugs. Uncontrolled trials produce higher estimates of the mean effect than those obtained in a controlled trial since, by not having a control group acting as a reference, uncontrolled trials can induce erroneous impressions of the investigated drug [7]. Sine these trials generate bias, the results of uncontrolled trials are considered less valid than those of controlled trials.
Controlled trials
The design of these trials includes at least one treatment group that is compared with a control group. The control group receives placebo or another active treatment. Both groups are studied simultaneously, except when the control group is derived from historical data or when some adaptive designs are used. Controlled trials are the most common in clinical phase III. Controlled trials allow the participant’s outcome to be discriminated from an outcome caused by other factors, such as the natural history of the disease or the expectations of the participant or the investigator.
Common controls are placebo control, active treatment control, control with dose comparison, and historical control. Particular care is required when attempting to use placebo control and historical control.
Placebo control
Placebo is defined as “an inert or innocuous substance used especially in a controlled experiment testing the efficacy of another substance (such as a drug)” [8]. This is especially useful if the outcome measured is subjective and should only be used if no permanent harm (death or irreversible morbidity) occurs by delaying available active treatment for the duration of the trial. The ethics of placebo-controlled studies is complex and continues to create a debate in the medical research community. According to the Declaration of Helsinki on the use of placebo released in October 2013, “the benefits, risks, burdens, and effectiveness of a new intervention must be tested against those of the best proven intervention(s), except in the following circumstances: where no proven intervention exists; the use of placebo, or no intervention, is acceptable; or where for compelling and scientifically sound methodological reasons the use of any intervention less effective than the best proven one, the use of placebo, or no intervention is necessary to determine the efficacy or safety of an intervention and the participants who receive any intervention less effective than the best proved one, placebo, or no intervention will not be subject to additional risks of serious or irreversible harm as a result of not receiving the best proven intervention. Extreme care must be taken to avoid abuse of this option” [9]. Hence, while designing a research study, both the scientific validity and ethical aspects of the study will need to be thoroughly evaluated.
Active treatment control
This design involves comparing a new drug with a standard drug or comparing the combination of new and standard therapies versus standard therapy alone. This design is more ethical than the placebo control, provided that approved drugs are available for the disease under study.
Control with dose comparison
Different doses or regimens of the same treatment are used as the active arm and control arm. The purpose is to establish a relationship between the dose and the efficacy and safety of the intervention. This design can include active and placebo groups in addition to the different dose groups. The design may be ineffective if the therapeutic range of the drug is not known.
Historical control (external and nonconcurrent)
In this design, the information from the controls is not obtained during the study but is from subjects who were treated at an earlier time or in a different setting. This design has an advantage when studying rare conditions in which difficulty arises in generating a sample size. This design is also cost-effective and time-saving. However, the design has many disadvantages. Randomization and blinding are not possible, and the comparability of the current intervention with the historical control is difficult due to the differences in baseline characteristics of the subjects. The comparability problem can be addressed to some extent by statistical methods, but the information obtained may not be accurate or reliable and may lack uniformity and/or completeness.
Randomized vs. nonrandomized clinical trials
Clinical trials are randomized or nonrandomized based on the method used to allocate a participant to a treatment or control group.
Randomized clinical trials
A randomized clinical trial involves randomizing participants with similar characteristics to one of two or multiple groups, the group(s) that receives the intervention/experimental therapy and the other group(s) that received the placebo or standard of care. Randomization is typically performed using a computer software package. Hence, we can measure the outcomes and efficacy of the intervention or experimental therapy being studied without bias as participants with similar baseline characteristics have been randomized to their respective groups. Randomized controlled trials are the gold standard for clinical study. However, this study design is generally not applicable to rare and serious disease processes due to the ethics involved in treating affected individuals with a placebo.
Nonrandomized trials
A nonrandomized trial involves an approach of selecting controls without randomization, usually allocation of participants into groups by the investigator. This may also result from selection of participants and controls based on day of the week presentation or assignment to a particular clinician. This type of participant and control selection becomes predictable. Therefore, there is bias introduced that can impact the validity of the results.
Open-label vs. blind trials
Clinical trials are divided into open-label versus blind trial based on participants’ or investigators’ awareness of the treatment group to which participants have been allocated.
Open-label trials
Certain treatments cannot be blinded such as surgeries or if the treatment group requires an assessment of the effect of intervention. In this case, open-label trials are planned in which both trial participants and investigators know the group assignment of the participants.
Blind trials
This is a method used in clinical trials to reduce the risk of intentional or unintentional bias. There are three forms of blinding: single, double, and triple blind. In a single-blind study, only the participants do not know their group assignment until the trial is over. In double-blind studies, both the study participants and the investigator are unaware of the group to which subjects were allocated. Double-blind studies are typically used in clinical trials to test the safety and efficacy of drugs. In triple-blind studies, participants, investigators, and data analysts are unaware of the group allocation. Those who are directly or indirectly involved in the trial, such as caregivers and data recorders, should also be blinded to the group allocation of the trial participant in order to increase the effect of blinding.
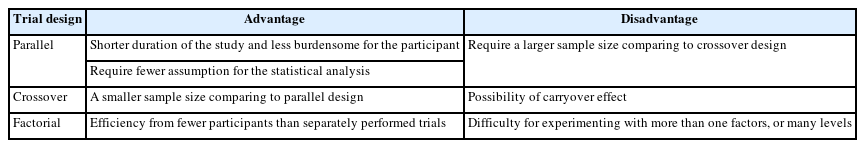
Parallel, crossover, and factorial design trials
Based on the treatment structure, clinical trial designs are classified into parallel, crossover, and factorial designs. A summary of the advantages and disadvantages of each design is provided in Table 2.
Parallel design trials
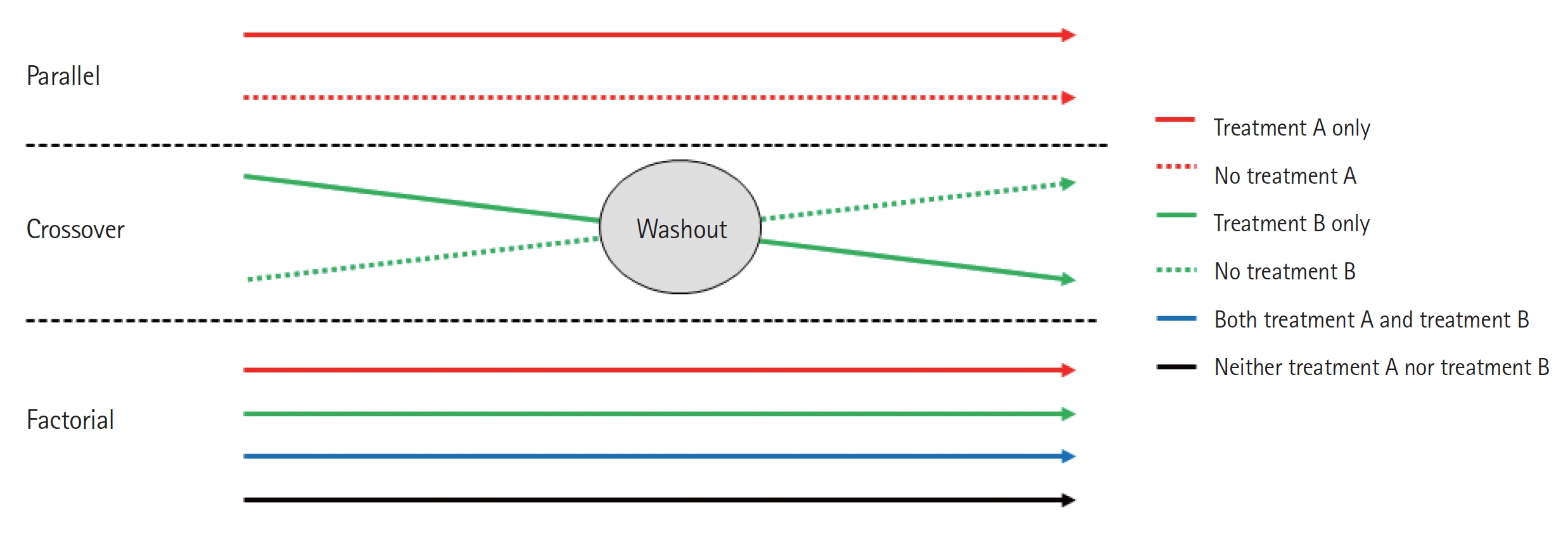
A parallel design of a clinical trial is a design in which two or more groups of participants receive different interventions. Participants are assigned to one of the treatment arms at the beginning of the trial and continue in that arm throughout the length of the trial (Fig. 3). This is the most common clinical trial design.
Parallel design has two advantages over the crossover design described later. All other conditions being the same, the duration of the study is shorter and the visits required are fewer, which results in a study that is less burdensome for the participant. The statistical analysis requires fewer assumptions, which, if not verified, would reduce the reliability of the conclusions. The weakness of the parallel design is that it requires a larger sample size than the crossover design.
Crossover design trial
The crossover clinical trial is a design in which all participants receive the same two or more treatments, but the order of receipt depends on the group of assignment. Hence, in this type of design, there are two groups that undergo the same intervention/experiment at different time periods. That is, each group serves as a control while the other is undergoing the intervention/experiment. A “washout” period is recommended in order to eliminate residual effects of the intervention or experiment (carryover effect) when the experiment group transitions to the control group, or vice versa (Fig. 3).
The main advantage of the crossover design is that each subject acts as a their own control. Therefore, a smaller number of subjects is required in comparison to parallel group studies because of removing of participant variation in this way. This type of trial can only be considered when the disease persists for a relatively long period. Hence, crossover trials are mostly used in studying chronic diseases. The main disadvantage is that the carryover effect may be aliased (confounded) with direct treatment effects as they cannot be estimated separately.
Factorial trial
In a factorial trial, two or more intervention comparisons are carried out simultaneously. For example, participants may be randomized to receive aspirin or placebo and randomized to receive a behavioral intervention or standard care. This factorial trial has two factors, each of which has two levels; there are called 2×2 factorial trials (Fig. 3). Whenc designing a factorial trial, the main intention of investigators is to achieve “two trials for the price of one”; and the assumptions are that the effects of the different active interventions are independent, and that there is no interaction (no synergy or antagonism) between the treatments. The interaction effect between the two treatments can be tested by a proper methodology. Since a 2×2 factorial trial can be seen as two trials addressing different questions, it is important that both parts of the trial are reported as if they were part of a two-arm parallel group trial. Thus, in the example given, we would expect to view the results for aspirin versus placebo, including all participants regardless of whether they had behavioral intervention or standard care, and likewise of the behavioral intervention. An evaluation of the interaction between the two treatments based on the factorial design may also be available.
The factorial design allows investigators to obtain evidence about efficacy from fewer patients than would be needed if treatment A and B were individually tested in two separate trials. The main disadvantage is the difficulty of experimenting with more than one factor or level. A factorial design must be planned meticulously, as an error in one of the levels, or in the general operationalization, will jeopardize a vast amount of work.
Pragmatic clinical trial design
A classical clinical trial may not be adequately reflective of practice because the trial may have been optimized to determine intervention efficacy. Because such trials were also performed with a relatively small size of highly selected participants at sites with experienced investigators, the trials could overestimate benefits and underestimate harm of the intervention. These concerns create the need for more pragmatic trials designed to demonstrate the actual effectiveness of the intervention in more generalized settings. Trial design can be more pragmatic when considering four domains: the study population, the setting of the trial, operationalization of the intervention, and the outcome measures [10,11]. In order to provide the comprehensive evaluation of comparative clinical effects of 0.9% saline and balanced crystalloids across the full spectrum of diseases typical for hospitalized adults, a pragmatic trial was conducted among noncritically ill adults who were subsequently hospitalized outside an ICU. This trial was designed to consider broad eligibility criteria, large sample size, study procedures that included routine care, and execution of the trial by clinical personnel [12,13].
CONCLUSION
The different types of clinical studies are used for different reasons. Selecting the best design for a given study is critical to a successful outcome. In terms of the quality of evidence, a clinical trial is superior to an observational study. Observational studies are, however, conducted much more frequently than clinical trials. Ethical considerations and cost are main reasons that observational studies are frequently employed. A case-control study is a valuable tool for exploring risk factors for rare diseases or when other types of study are not feasible. Investigators explore possible associations between exposure and disease through case-control studies, and data from case-control studies can provide a focus for future studies. Then, through cohort studies or clinical trials, the evidence of an association between exposure and disease can be increased. Cohort studies are often complex, large, and long in duration. However, with careful planning and implementation, cohort studies are valuable in providing healthcare evidence. To reduce cost and achieve the same goal as a cohort study, nested case-control and case-cohort study are alternatives. These types of studies are based on large cohorts and can be useful in “big data” analysis [14]. Nested case-control and case-cohort study designs are efficient in terms of cost and can be used to evaluate the relationship between exposure and disease. Compared to a nested case-control design, the case-cohort design is more efficient and allows an investigator to study several disease outcomes using the same random sample [15]. While there are some advantages in observational studies, biases are inherent and should be addressed. Recently, as studies using “big data” have become possible, well-designed historical control studies have increased. In clinical trials, appropriate control group selection is vital. The clinical trial study should be planned so that those involved in the study, including participants, are blinded to the maximum extent possible. The classical trial designs are parallel, crossover, and factorial designs. In addition, although not applicable to all diseases or clinical trials, new methodologies such as adaptive designs can shorten the duration of a clinical trial. Investigators should also consider pragmatic clinical trials that are more efficient, patient-centered, and empirical and are conducted in order to provide more valuable clinical and policymaking information.
Notes
Conflicts of interest
The author has no conflicts of interest to declare.
Funding
The author received no financial support for this study.
Data availability
Data sharing is not applicable as no new data were created or analyzed in this study.
References
Article information Continued
Notes
Capsule Summary
What is already known
Multiple study designs exist to help determine the factors associated with a disease and to assess the efficacy and safety of an intervention.
What is new in the current study
In this paper we review multiple study designs and discuss their advantages and disadvantages.