Hemodynamic management of septic shock: beyond the Surviving Sepsis Campaign guidelines
Article information
Abstract
Although the Surviving Sepsis Campaign guidelines provide standardized and generalized guidance, they are less individualized. This review focuses on recent updates in the hemodynamic management of septic shock. Monitoring and intervention for septic shock should be personalized according to the phase of shock. In the salvage phase, fluid resuscitation and vasopressors should be given to provide life-saving tissue perfusion. During the optimization phase, tissue perfusion should be optimized. In the stabilization and de-escalation phases, minimal fluid infusion and safe fluid removal should be performed, respectively, while preserving organ perfusion. There is controversy surrounding the use of restrictive versus liberal fluid strategies after initial resuscitation. Fluid administration after initial resuscitation should depend upon the patient’s fluid responsiveness and requires individualized management. A number of dynamic tests have been proposed to monitor fluid responsiveness, which can help clinicians decide whether to give fluid or not. The optimal timing for the initiation of vasopressor agents is unknown. Recent data suggest that early vasopressor initiation should be considered. Inotropes can be considered in patients with decreased cardiac contractility associated with impaired tissue perfusion despite adequate volume status and arterial blood pressure. Venoarterial extracorporeal membrane oxygenation should be considered for refractory septic shock with severe cardiac systolic dysfunction.
INTRODUCTION
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock is a subset of sepsis in which underlying circulatory, cellular, and metabolic abnormalities are profound enough to substantially increase mortality [1]. Despite advances in sepsis care, sepsis remains a major cause of morbidity and mortality worldwide, including in Korea. In 2017, an estimated 48.9 million sepsis cases were recorded worldwide, and 11.0 million sepsis-related deaths were reported globally, representing 19.7% of all global deaths [2,3].
To reduce the morbidity and mortality associated with sepsis, the Surviving Sepsis Campaign (SSC) guidelines were first launched in 2002 by the European Society of Intensive Care Medicine (ESICM), the International Sepsis Forum (ISF), and the Society of Critical Care Medicine (SCCM). Since then, these guidelines have been regularly updated based on new research findings and clinical experiences [4]. While the guidelines provide standardized and generalized guidance, they are supported by evidence mainly based on randomized controlled trials (RCTs) that investigated patient response to a single intervention. However, recent large RCT studies have failed to show a difference in mortality [5–7]. The reason for this is that these RCT studies did not consider the characteristics of individual patients that may affect their response to specific interventions. Given that sepsis is a complex condition with variable clinical courses, patient phenotypes, and treatment responses, a “one-size-fits-all” management approach may not be appropriate for all patients. In this respect, the evidence-based SSC guidelines appear to be less individualized. Future sepsis treatment should be individualized based on the diversity of sepsis. This narrative review focuses on recent updates in the personalized hemodynamic management of septic shock not covered in the SSC guidelines.
PERSONALIZED HEMODYNAMIC MANAGEMENT
Hemodynamic support remains a cornerstone in the management of septic shock. Different phases exist in the management of shock, including the salvage, optimization, stabilization, and de-escalation phases [8], and monitoring and intervention should be personalized and tailored according to the phase of shock (Fig. 1) [9].

Hemodynamic monitoring, targets, and interventions at the different phases of shock. CRT, capillary refill time; MAP, mean arterial pressure; DAP, diastolic arterial pressure; SvO2, mixed venous oxygen saturation; ScvO2, central venous oxygen saturation; Pv-aCO2, venous-to-arterial carbon dioxide difference; TPTD, transpulmonary thermodilution; EVLW, extravascular lung water.
Personalized hemodynamic monitoring
Salvage phase
In the salvage phase, the goal of treatment is to provide life-saving tissue perfusion. A mean arterial pressure (MAP) of ≥65 mmHg and diastolic arterial pressure (DAP) of ≥45 mmHg should be achieved. Clinical assessment can identify patients who may respond to fluids and assess their response [10]. Altered clinical signs, including hypotension, tachycardia or bradycardia, cold extremities, skin mottling, increased capillary refill time (CRT), and oliguria, are important warning signals indicating that tissue hypoperfusion is occurring, but these signs cannot reliably indicate whether the cardiac output (CO) is low or high nor indicate the source of the hemodynamic alteration [11]. For this purpose, physicians should perform additional evaluations, such as lactate measurements and echocardiography. If cardiac impairment is suspected or the patient fails to respond to fluid therapy, bedside echocardiography is the only useful tool for rapid estimation of cardiac dysfunction along with the identification of the cause of low CO. Blood lactate level measurement is also useful for identifying impairments in tissue perfusion [9].
Optimization phase
The primary goal during the optimization phase is to optimize tissue perfusion. In addition to the monitoring tools used in the salvage phase, central venous oxygen saturation (ScvO2) or mixed venous oxygen saturation (SvO2) and venous-to-arterial carbon dioxide difference (Pv-aCO2) measurement may be used to estimate tissue perfusion [9]. ScvO2 or SvO2 reflects the balance between the actual oxygen consumption and tissue oxygen delivery. A low ScvO2 indicates inadequate oxygen delivery if hemoglobin and arterial oxygen saturation values are within normal ranges [9]. Pv-aCO2, defined as the difference between the venous and arterial carbon dioxide partial pressures, is inversely related to CO. Increased Pv-aCO2 reflects decreased microvascular blood flow during early phases of resuscitation in septic shock [12]. It is important to note that there are differences in the normalization rate between monitoring tools. In an observational study, monitoring tools such as ScvO2, Pv-aCO2, and CRT were already normal in >70% of survivors at 6 hours, whereas lactate showed a much slower normalization rate, decreasing significantly at 6 hours compared to baseline but with only 52% of patients achieving normality at 24 hours [12]. Therefore, it is preferable to use several monitoring tools in combination rather than just a single one.
Transpulmonary thermodilution, an advanced monitoring tool, allows continuous and real-time monitoring of CO. It estimates the end-diastolic volume and systolic function of the four cardiac chambers. It also measures extravascular lung water (EVLW), which quantifies the volume of pulmonary edema, and pulmonary vascular permeability, which quantifies the degree of a pulmonary capillary leak [13,14]. Transpulmonary thermodilution should be considered in patients with severe septic shock.
Stabilization phase
In the stabilization phase, the goal is to preserve organ perfusion and prevent organ dysfunction. Cardiac dysfunction and volume overload are common in this stage, and hemodynamic tools already in use can continue to be used. In particular, repeated echocardiography may be helpful to uncover the development of right ventricular dysfunction [9].
De-escalation phase
Finally, in the de-escalation phase, the goal is to achieve a negative fluid balance by weaning patients off vasoactive drugs and promoting spontaneous polyuria or by inducing fluid clearance using diuretics or ultrafiltration. Monitoring can be minimized. Tissue perfusion and fluid responsiveness should be evaluated prior to fluid removal. When hypoperfusion occurs, de-escalation should be stopped [9].
Fluid management after initial resuscitation
For patients with sepsis-induced hypoperfusion or septic shock, the SSC guidelines suggest that ≥30 mL/kg of intravenous crystalloid fluid should be given within the first 3 hours of resuscitation [4]. This fixed volume during initial resuscitation was chosen mainly based on the results of several large RCT trials [5–7,15–17].
However, the SSC guidelines suggest no recommendation for fluid administration in patients with sepsis and septic shock who still have signs of hypoperfusion and volume depletion after initial resuscitation and that fluid resuscitation should be given only if patients present with signs of hypoperfusion. The guidelines emphasize that fluid administration after the initial fluid bolus should be guided by perfusion parameters as well as a response in hemodynamic variables [4]. Liberal fluid administration may have detrimental effects by causing edema in vital organs, leading to organ dysfunction and impairment of oxygen delivery, but the restrictive fluid strategy primarily relies on vasopressors to reverse hypotension and maintain perfusion while limiting fluid administration [18]. Observational clinical studies and randomized trials have reported harmful effects, including kidney injury, respiratory failure, or high mortality. These studies suggest that a restrictive fluid strategy is potentially superior to a liberal fluid strategy [19–23]. Recently, the results of two RCT studies related to restrictive versus liberal fluid strategies after initial resuscitation have been published. In the CLASSIC (Conservative vs. Liberal Approach to fluid therapy of Septic Shock in Intensive Care) trial [24], the restrictive fluid group received an intravenous fluids bolus of 250 to 500 mL if the patient had severe hypoperfusion, which was defined as a plasma lactate value of ≥4 mmol/L, a MAP of <50 mmHg despite infusion of a vasopressor or an inotropic agent, a mottling score >2 points (on a scale of 0–5 points, with higher scores indicating a greater area of mottling), or a urinary output of <0.1 mL/kg/hr during the first 2 hours after randomization. In the standard fluid group, no upper limit of fluid administration was set. The study found that intravenous fluid restriction did not cause fewer deaths at 90 days than standard intravenous fluid therapy. Separately, in the CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis) trial [25], patients with sepsis-induced hypotension refractory to initial treatment with 1 to 3 L of intravenous fluid were enrolled. There was no difference in 90-day mortality or adverse outcomes between groups receiving the restrictive fluid strategy (prioritizing vasopressors and lower intravenous fluid volumes) and the liberal fluid strategy (prioritizing higher volumes of intravenous fluids before vasopressor use), respectively. These studies showed that restrictive fluid therapy is not superior to liberal fluid therapy. This means that fluid administration after initial resuscitation may vary depending on the patient’s fluid responsiveness and require individualized management. A comprehensive evaluation, including tissue perfusion monitoring, benefits and risks of fluid infusion, and fluid responsiveness, should be completed to achieve individualized fluid management, which should be preferred over a restrictive or liberal fluid strategy [13].
Tests to predict fluid responsiveness
The goal of fluid administration in patients with septic shock is to increase CO and tissue perfusion. However, fluid infusion can cause deleterious effects of fluid overload without an increase in CO. In an observational cohort study [26], only two-thirds of patients with septic shock were fluid-responders. Therefore, patients not responding to volume expansion may experience fluid overload [27]. Fluid overload has been shown to cause enhanced shedding of the endothelial glycocalyx, whose disruption increases vascular permeability, leading to tissue edema [28]. To prevent harmful effects of fluid overload, predicting fluid responsiveness should be the first step of a fluid strategy. Fluid responsiveness refers to a set of bedside tests that reversibly increase the preload status of the heart, allowing the clinician to assess whether this manipulation determines a significant increase in CO [29]. Fluid responsiveness is commonly defined as a stroke volume (SV) increase of ≥10% following a fluid bolus of 200 to 500 mL in 10 to 15 minutes [30,31]. For this purpose, static measurements of preloads, including central venous pressure, inferior vena cava diameter, and arterial pressure, have been used for decades but are unreliable. Strong evidence suggests that these traditional uses should be abandoned [30–34]. In the last two decades, a number of dynamic tests have been proposed to establish and monitor fluid responsiveness (Table 1). These dynamic tests use heart-tolung interactions, passive leg raising, or mini-fluid challenges to induce short-term changes in cardiac preloads and reveal their effects on CO [30,35]. All have some limitations, but they are frequently complementary, which helps clinicians to make the decision to give fluid or not [30,35].
In 2018, an expert statement [36] proposed an individualized fluid treatment based on a repeated bolus of 250 to 500 mL of intravenous crystalloids with the continuous monitoring of fluid responsiveness and the early administration of vasopressors if circulation fails to improve. Since it is impractical to standardize the amount of fluid according to each patient, an individualized strategy of resuscitation based on fluid responsiveness is preferable.
On the other hand, fluid unresponsiveness could be used to safely remove fluids in the hemodynamically stable patient [37].
TIMING OF VASOACTIVE AGENT INITIATION IN SEPTIC SHOCK
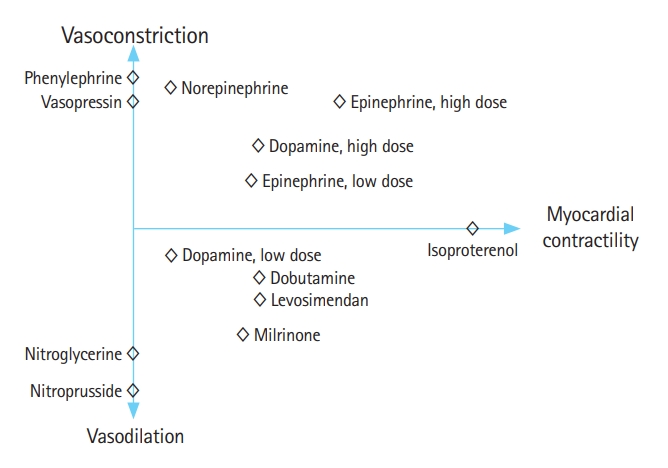
Septic shock results in shedding of the vascular endothelial glycocalyx and endothelial damage, which leads to increased permeability, diffuse alterations in microvascular perfusion, and vasodilation due to a marked decrease in vascular tone [28]. Hypotension in patients with septic shock is known to be associated with increased mortality [37,38]. Vasoactive agents play a crucial role in septic shock management by modulating vascular tone and enhancing myocardial contractility. Vasoactive agents possess varying abilities to constrict or dilate blood vessels and enhance myocardial contractility (Fig. 2) [39]. The selection of vasoactive agents is tailored to the individual patient’s hemodynamic profile and specific needs to achieve optimal cardiovascular stability and tissue perfusion. The SSC guidelines recommend norepinephrine as a first-line vasopressor to maintain a target MAP of 65 mmHg for initial resuscitation [4]. Norepinephrine is both an α-1 and β-1 adrenergic agonist that predominantly enhances vascular filling pressure and redistributes blood flow via its vasoconstrictive effect and myocardial contractility [40]. In septic shock patients, a decrease in norepinephrine dose led to a more significant decrease in mean systemic pressure than a decrease in resistance to venous return, leading to a reduction in venous return [41].
Timely initiation of vasopressors with fluid resuscitation is a key component in the management of septic shock. However, the optimal timing for the initiation of vasopressors has not been known. There are no recommendations on the timing of vasoactive agent initiation for septic shock treatment in the SSC guidelines. Recent data showed an association between delayed therapy and increased mortality and suggested that early initiation of vasopressors should be considered [37,42,43]. The 2018 SSC hour-1 bundle, which recommends vasopressor therapy within the first hour during or after volume resuscitation if blood pressure is not restored after initial fluid resuscitation to achieve a MAP of ≥65 mmHg [44]. In a retrospective study, every 1-hour delay in norepinephrine initiation during the first 6 hours after septic shock onset was associated with a 5.3% increase in mortality. Mortality rates at 28 days were significantly higher when norepinephrine administration was started ≥2 hours after septic shock onset compared to <2 hours [42]. A very early start of vasopressors within/before the next hour of the first resuscitative fluid load was related to a significant lower net fluid balance and also with a significant reduction in the risk of death at 28 days [45].
Early high-dose vasopressor within the first 6 hours of shock is associated with lower mortality [46]. In a systematic review and meta-analysis, early initiation of norepinephrine in patients with septic shock was associated with decreased short-term mortality, a shorter time to achieve the target MAP, and a smaller volume of intravenous fluids within 6 hours [47]. In the CENSER (Early Use of Norepinephrine in Septic Shock Resuscitation) trial [48], a single-center, prospective, double-blind, placebo-controlled trial, the early vasopressor group received norepinephrine at 1.5 hours compared to 3 hours in the standard treatment group. The shock control rate at 6 hours, which was the primary endpoint, was met in 76.1% of patients in the early vasopressor group compared to 48.4% of patients in the standard group (P<0.001), while there was no difference in 28-day mortality between these groups.
In contrast, earlier vasopressor use with a restrictive fluid strategy compared to later vasopressor use with a liberal fluid strategy did not result in significantly lower (or higher) mortality before discharge home by day 90 [25]. Similarly, vasopressor initiation within 1 hour of fluid loading was associated with higher 28-day mortality in patients with septic shock [49].
DAP and the diastolic shock index (DSI), defined as the ratio between heart rate and DAP, may be used to guide the timing of vasopressor initiation in septic shock. It seems logical to initiate vasopressors when DAP <45 mmHg or DSI >2, which indicates severe vasodilation [50]. A retrospective observational study showed that in patients with high DSIs (≥2.0) and high lactate levels (≥2.5 mmol/L), early initiation of vasopressor therapy was associated with decreased 28-day mortality [51]. These data suggest that norepinephrine should be initiated early, ideally within 1 hour of shock onset, but after adequate fluid resuscitation. DSI and lactate measurement can help guide the appropriate time to initiate vasopressor therapy in septic shock [52].
The SSC guidelines suggest adding vasopressin instead of escalating the dose of norepinephrine in adults with septic shock on norepinephrine with inadequate MAP levels. However, the timing of vasopressin initiation is not well-described in the literature. In VASST (Vasopressin and Septic Shock Trial) [53], there was no difference in 28-day mortality, but subgroup analyses identified a mortality benefit with the use of vasopressin in patients with less severe septic shock, i.e., those with a norepinephrine dose at randomization of ≤15 μg/min and those with a lactate concentration at randomization of ≤1.4 mmol/L. In a retrospective, observational study, a greater norepinephrine-equivalent dose at vasopressin initiation and a higher lactate concentration at vasopressin initiation were each associated with higher in-hospital mortality in patients with septic shock [54]. These data indicate that vasopressin should be initiated when patients are on low norepinephrine-equivalent doses or have low lactate concentrations. While the SSC guidelines suggest vasopressin initiation when the norepinephrine dose is in the range of 0.25 to 0.5 μg/kg/min [4], vasopressin initiation may be considered before norepinephrine-equivalent doses exceed 0.1 to 0.2 μg/kg/min (10–15 μg/min) [52].
Epinephrine should be considered as a third-line treatment for septic shock, and its use should be limited to those patients with inadequate MAP levels despite norepinephrine and vasopressin administration [4]. The specific norepinephrine-equivalent dose at which epinephrine should be administered in septic shock is unknown. One study [55] identified the optimal norepinephrine-equivalent dose range for initiating epinephrine as 37 to 133 μg/min. In this dose range, 29% of patients achieved hemodynamic stability with the initiation of epinephrine, while 15% of patients who had epinephrine initiated outside of this dose range achieved hemodynamic stability (P=0.03).
INOTROPES
Sepsis-induced cardiomyopathy (SCM) is a reversible myocardial dysfunction caused by sepsis. The prevalence of SCM varies from 10% to 70%, although studies defining SCM as an ejection fraction of <45% have generally reported a prevalence of 30% to 50% [56]. Inotropes can be considered in patients with decreased cardiac contractility associated with impaired tissue perfusion. The SSC guidelines suggest either adding dobutamine to norepinephrine or administering epinephrine alone for adults with septic shock and cardiac dysfunction with persistent hypoperfusion despite adequate volume status and arterial blood pressure [4]. Adverse effects (tachyarrhythmia, increased heart rate, hypotension, and myocardial oxygen consumption) and specific risks (hypertrophic cardiomyopathy, myocardial ischemia) should be carefully investigated, and the risk/benefit profile of intervention should be evaluated [9]. Milrinone is a phosphodiesterase inhibitor that increases intracellular cyclic adenosine monophosphate, leading to inotropic effects independent of β-adrenergic receptors [15]. Milrinone may be an effective therapeutic in patients recently on β-blockers [57]. Experts suggest adopting a stepwise approach to administering inotropics, as follows. First, begin with a limited dose of dobutamine (2.5–5.0 μg/kg/min) and evaluate efficacy and tolerance. If there is still severe contractility impairment, higher doses (≤20 μg/kg/min) may be considered. Second, substitute or add enoximone or milrinone and evaluate the efficacy and tolerance. Third, substitute or add levosimendan in cases of severe impairment. At each step, improvements in cardiac function and CO as well as resolution of tissue hypoperfusion and tolerance (e.g., lack of tachycardia, arrhythmias, etc.) should be evaluated. As soon as the situation improves, weaning off inotropics should be attempted [9]. However, the SSC guidelines suggest against using levosimendan, as it was not superior to dobutamine in adults with sepsis in terms of mortality [4,58].
TIMING OF CORTICOSTEROID INITIATION IN SEPTIC SHOCK
Sepsis results in disruption of the hypothalamic-pituitary-adrenal axis, which may translate into cardiovascular and other organ dysfunction and eventually an increased risk of death. Corticosteroids are known to improve cardiovascular function via sodium and water retention, restore systemic vascular resistance, and decrease organ failure [59]. Three recent large RCTs [60–62] showed that corticosteroids accelerate the resolution of shock, but there was no clear effect on short- or long-term mortality. The SSC guidelines suggest administering intravenous corticosteroids in patients with septic shock and ongoing requirements for vasopressor therapy [4].
Although there is no clear recommendation with regard to the time of initiation of corticosteroids in septic shock patients, the early initiation of corticosteroid therapy in sepsis, specifically within 24 hours of shock, despite adequate fluid resuscitation and vasopressor administration (norepinephrine-equivalent dose of 0.5–1 μg/kg/min) is reasonable [52]. A retrospective cohort study reported decreased intensive care unit mortality when hydrocortisone was administered within 0 to 6 hours after shock onset compared to >48 hours after shock onset (odds ratio, 0.6; 95% confidence interval 0.4–0.8) and suggested that hydrocortisone should be started within the first 12 hours after shock onset [63].
A recent multicenter, propensity score-weighted observational cohort study (n=198) [64] evaluated early (≤12 hours of vasopressor initiation) versus late (>12 hours of vasopressor initiation) low-dose corticosteroid initiation in septic shock and determined that early initiation was associated with a shorter time to vasopressor discontinuation (40.7 hours vs. 60.6 hours, P=0.0002). The SSC guidelines suggest that corticosteroid administration is commenced ≥4 hours after vasopressor initiation and at norepinephrine or epinephrine doses of ≥0.25 μg/kg/min [4].
VA ECMO IN SEPTIC SHOCK
While the SSC guidelines suggest using venovenous extracorporeal membrane oxygenation (ECMO) when conventional mechanical ventilation fails for sepsis-induced severe acute respiratory distress syndrome, there is no suggestion of deploying venoarterial (VA) ECMO in septic shock complicated by SCM [4]. Most early studies of VA ECMO for refractory septic shock complicated by SCM reported low survival rates and poor outcomes [65]. A recent retrospective, multicenter study [66] showed that patients with severe sepsis-induced cardiogenic shock treated with VA ECMO experienced a large and significant improvement in survival compared to controls not receiving ECMO (60% vs. 25%, P<0.0001). A meta-analysis of 468 patients placed on VA ECMO for refractory septic shock [67] reported an overall survival rate of 36% and a significantly higher survival rate among patients with ejection fractions of <20% compared to those with ejection fractions of >35% (62% vs. 32.1%, P=0.05). Therefore, VA ECMO should be considered as a bridge therapy to recovery for patients with refractory septic shock with severe cardiac systolic dysfunction and end-organ hypoperfusion. However, VA ECMO should not be used to manage patients with isolated vasodilatory septic shock without significant myocardial dysfunction [65].
CONCLUSION
Sepsis is a complex condition with variable clinical courses, patient phenotypes, and treatment responses. Personalized hemodynamic monitoring and fluid responsiveness based on the phase of septic shock are essential in septic shock management to assess the patient’s cardiovascular status, guide fluid resuscitation, determine the need and timing for vasopressors and inotropic agents, and optimize tissue perfusion, which leads to improved outcomes in septic shock.
Notes
ETHICS STATEMENT
Not applicable.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: GJS; Data curation: GJS; Methodology: all authors; Visualization: all authors; Writing–original draft: GJS; Writing–review & editing: all authors. All authors have read and approved the final manuscript.
References
Article information Continued
Notes
Capsule Summary
What is already known
The Surviving Sepsis Campaign guidelines provide standardized and generalized guidance based on randomized controlled trials that investigated patient responses to a single intervention. Sepsis is a complex condition with variable clinical courses, patient phenotypes, and treatment responses. Therefore, a “one-size-fits-all” management approach based on current guidelines may not be appropriate for all patients.
What is new in the current study
Hemodynamic monitoring and fluid management should be personalized according to the phase of shock. There is controversy surrounding the use of restrictive versus liberal fluid strategies after initial resuscitation. Fluid administration after initial resuscitation should be determined by the patient’s fluid responsiveness. Recent data suggest early initiation of vasopressors if blood pressure is not restored after initial fluid resuscitation. Venoarterial extracorporeal membrane oxygenation can be considered for refractory septic shock with severe cardiac systolic dysfunction.