Tranexamic acid for angiotensin-converting enzyme inhibitor–induced angioedema
Article information
Abstract
Approximately 0.7% of patients taking angiotensin-converting enzyme inhibitors (ACEIs) develop ACEI-induced angioedema (ACEI-IA). With no approved treatments for ACEI-IA, the risk of complications is concerning. Tranexamic acid (TXA) has the potential to prevent intubations and resolve ACEI-IA by inhibiting the downstream production of bradykinin. In this review, we aim to evaluate the safety and efficacy of TXA use in ACEI-IA. We queried the PubMed database for studies involving TXA for ACEI-IA from January 2003 to January 2023. Seven studies met the study inclusion criteria. Our results demonstrate that TXA may improve angioedema symptoms and prevent intubation. In addition, its availability, low cost, and safety profile support its use for improving the symptoms and complications of ACEI-IA in an emergency setting.
INTRODUCTION
Approximately 30% to 40% of all angioedema-related emergencies are caused by angiotensin-converting enzyme inhibitor (ACEI) medications [1]. The populations with the highest risk of developing ACEI-induced angioedema (ACEI-IA) are women and African Americans, who are nearly 4.5 times more likely to develop ACEI-IA [2,3].
ACEI-IA, a potentially fatal complication of ACE inhibition, occurs in up to 0.7% of patients treated with ACEIs. The incidence of ACEI-IA is the highest within the first month of therapy, accounting for nearly one-third of all cases [4]. However, the onset of ACEI-IA can occur any time after initiation and has been reported as late as 20 years after treatment commences [5]. Lisinopril is the most common causative agent in 87.2% of reported ACEI-IA cases, with lower rates reported in other ACEIs such as enalapril 4.3% and benazepril 3.0% [3,6].
CLINICAL MANIFESTATIONS AND CURRENT TREATMENT
The pathophysiology of angioedema involves a rapid increase in vascular permeability and subsequent submucosal edema. With ACE inhibition, the reduced kininase II mediated degradation of substance P and bradykinin causes excessive vasodilation and plasma extravasation [4,7].
An attack of ACEI-IA typically lasts 48 to 72 hours, and patients require hospital admission in most cases [7,8]. Nearly 10% of all ACEI-IA cases require intubation within the first 6 hours of symptom onset [9]. In addition, approximately 40% of ACEI-IA patients are admitted to the intensive care unit for an average length of 2.2 days [9,10]. First-line treatment for ACEI-IA is an immediate cessation of ACEI and active airway management.
Given the prevalent use of ACEIs, there is an urgent clinical need for a rapidly effective therapeutic intervention for severe ACEI-IA. The successful use of tranexamic acid (TXA) for the prophylaxis of acquired angioedema was first reported in an emergency setting by Beauchene et al. [11] in 2018. This review aims to investigate the efficacy of TXA use in the acute management of ACEI-IA.
LITERATURE REVIEW
We searched the literature for TXA and ACEI-IA in the PubMed database with the following search terms: “tranexamic acid for bradykinin angioedema” and “tranexamic acid for angiotensin-converting enzyme inhibitor-induced angioedema.” The results were limited to observational studies, case reports, case series, and literature reviews published within the last 20 years and resulted in 54 eligible studies. After excluding studies evaluating tranexamic use in hereditary angioedema or non–bradykinin-mediated angioedema, there were seven articles for full-text review.
Included parameters of interest were study name, study type, sample size, primary outcomes, and results. The key findings of the search results are listed in Table 1 [11–17]. Key primary endpoints of interest included the percentage of patients with resolved or improved angioedema, overall intubation rates, and treatment-related adverse effects. For treatment maintenance studies, the frequency of acute angioedema attacks, treatment efficacy, and treatment safety parameters were included.
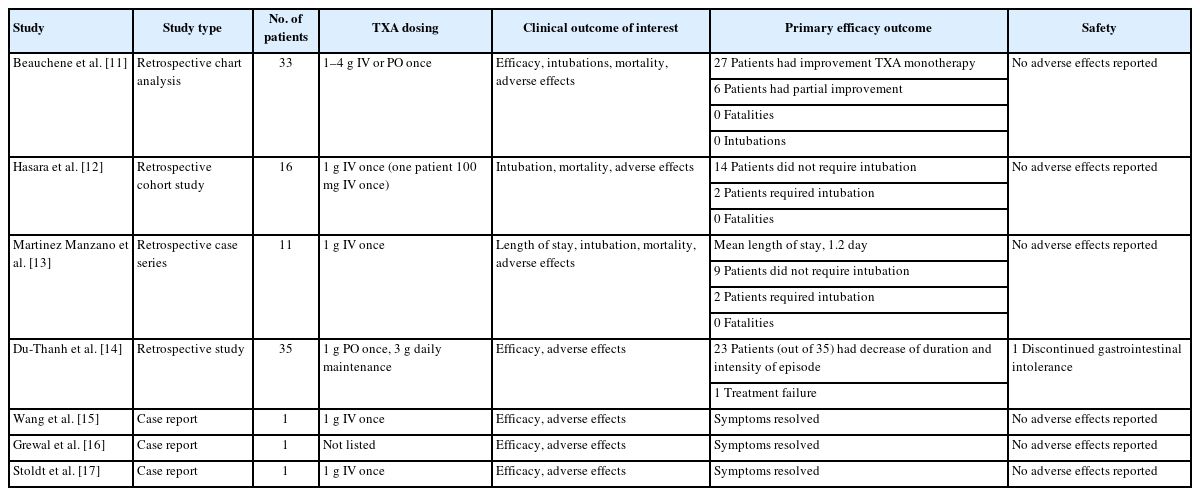
Findings of selected studies for TXA use in angiotensin-converting enzyme inhibitor-induced angioedema
Seven studies that met the inclusion criteria were identified: three were retrospective, one was a case series, and three were individual case reports. All studies commented on the general efficacy of TXA for bradykinin-mediated angioedema conditions and included information regarding intubation, mortality, and adverse effects.
The two largest retrospective studies [11,12] consisted of 33 and 16 patients, respectively. The primary outcomes were the time to the resolution of angioedema symptoms and the number of patients that required intubations. In the larger 33-patient retrospective cohort study [11], TXA was used as first-line therapy, with most patients (81.81%) showing significant improvement (regression of edema/dyspnea or other symptoms, not complete remission) with TXA treatment alone. Additional treatment with icatibant was required in 15.2% of patients and 3.0% needed C1 esterase inhibitor (C1-INH) concentrate after a partial resolution of symptoms with TXA monotherapy, suggesting some patients did not have an adequate resolution of symptoms. In addition, approximately 40% of patients reported improvement within 1 hour of TXA administration and there were no intubations, and no side effects or fatalities occurred. In the smaller 16-patient retrospective cohort study [12], 87.5% of the patients did not require intubations, with the other 12.5% receiving intubation before TXA administration. No patients reported worsening angioedema in this study, with 74% of patients experiencing partial resolution of symptoms following TXA infusion. The patients reported no adverse effects in this study as well.
A retrospective case series of 11 ACEI-IA patients treated with TXA [13] reported the median length of hospital stay was 1.2 days. Although this series reported two intubation cases, both patients were intubated before the administration of TXA. The mortality rate in this case series was 0%, with no reported adverse effects.
Another study [14] included 35 patients, of which 25 received TXA during an episode of bradykinin-related angioedema. TXA was well tolerated and led to at least a partial resolution of symptoms in 92% of patients, with 48% having complete resolution. Treatment failure and treatment discontinuation due to digestive intolerance occurred in two patients, respectively. Four patients were not treated with TXA due to thromboembolic contraindications. Although this study was not specific to ACEI-IA, a similar underlying mechanism in angioedema supports the pharmacological action of TXA in bradykinin-mediated angioedema [13].
In addition to the retrospective studies conducted, three additional case reports [15–17] have been published on TXA use in ACEI-IA. At least partial resolution of symptoms with no adverse effects, intubations, or mortality was reported. Additionally, two clinical studies [12,13] reported two cases, each requiring intubation due to ACEI-IA, but these patients were both intubated before TXA initiation.
DISCUSSION
The mechanism of action for TXA in the treatment of ACEI-IA is not well understood. However, blockage of plasmin activation by TXA contributes to its antifibrinolytic effect and is an important step in amplifying kallikrein (a precursor of bradykinin) activation. TXA prevents fibrin-induced inflammatory peptides and decreases the conversion of kininogen into bradykinin. C1 esterase activates plasma kallikrein and factor XIIa to allow downstream bradykinin development [8,18]. Further research is needed to clarify the exact mechanisms by which TXA exerts its therapeutic effects in this population.
There are currently no US Food and Drug Administration (FDA)-approved medications for treating ACEI-IA, and no current guideline recommendations are available for acute ACEI-IA treatment. Traditional treatment of ACEI-IA revolves around discontinuing the offending agent and providing symptom management. Agents that are traditionally used for “on demand” treatment of hereditary angioedema (HAE) include ecallantide, icatibant, plasma-derived nano-filtered C1-INH, and recombinant C1-INH with limited studies in the acute treatment of ACEI-IA. In line with the mechanism of ACEI-IA, ecallantide inhibits kallikrein, icatibant blocks bradykinin, and C1-INH inhibits the activity of C1 esterases [19–21].
Untreated angioedema can progress to a compromised airway and without acute management can lead to increased mortality [22,23]. Given the findings of recent retrospective cohort studies and case reports, TXA has shown clinical utility in an adequate resolution of symptoms and preventing angioedema progression to intubation. Our review suggests that most patients had partial or complete resolution of symptoms following TXA treatment, with few requiring intubation [11–17,24].
An additional clinical study evaluated TXA use as a maintenance treatment for nonhistaminergic angioedema in 37 patients, of which 18 were diagnosed with HAE and 19 were diagnosed with idiopathic angioedema [24]. These patients also did not respond to antihistaminic treatment (even at high doses) and complement treatment was not explored. Of the 19 patients, there were only three acute angioedema attacks in 6 months following TXA initiation, of which only one was severe. There were no cases of an increased number of attacks before TXA initiation. There were six accounts of adverse effects, of which 66.66% were digestive adverse effects and 33.33% were dizziness [24]. Although these patients were not confirmed to have ACEI-IA, it suggests the potential of TXA for nonhistaminergic angioedema in confirmed cases of non-HAE. The higher prevalence of adverse effects can be attributed to routine prophylactic use; however, in the emergency setting for ACEI-IA, TXA is administered less frequently.
The dosage of intravenous TXA in studies varied from 500 mg to 4 g administered per event with most patients receiving 1 g intravenously [11–17,24]. This is similar to the typical prophylactic dose for HAE of 1 g twice daily in adults [25]. Additionally, TXA has a favorable safety profile, with many studies reporting no adverse effects after an acute infusion [12,14]. A pooled analysis of two randomized controlled trials over 947 cycles in 500 women for heavy menstrual bleeding found that subjects using oral TXA at 3,900 mg/day experienced at least one adverse reaction compared to placebo (208 of 232 [89.7%] vs. 139 of 122 [87.8%]) [26]. The most common adverse events include headache (50.4% vs. 46.8%), nasal and sinus symptoms (25.4% vs. 17.3%), and back pain (20.7% vs. 15.1%) [26]. While no thromboembolic events were reported in the articles evaluated, it is important to consider the benefit of avoiding intubation versus the potential risk of thrombotic events.
OTHER THERAPEUTIC AGENTS
Other currently used off-label treatment options for ACEI-IA include agents used commonly for hereditary angioedema, including icatibant, ecallantide, fresh frozen plasma infusions, and C1-INH. Icatibant, a competitive bradykinin B2 receptor antagonist, is FDA-approved for the treatment of acute attacks of HAE. However, studies of overall efficacy in its use in ACEI-IA are mixed [19,27]. A potential benefit of icatibant is that it does not require hepatic or renal impairment dose adjustment [28]. Thus, there is limited data supporting icatibant use in ACEI-IA. In addition, its relatively high cost and limited availability make it less appealing for emergency use [29].
Ecallantide is FDA-approved for HAE and has been used off-label for ACEI-IA with mixed efficacy data because there is no statistical difference against the placebo for improving discharge criteria [20,30]. Ecallantide is a parental recombinant protein inhibitor of kallikrein thought to decrease bradykinin production to resolve angioedema. Similar to icatibant, ecallantide has a relatively high cost and limited availability, bringing into question its clinical effectiveness in medical practice [31]. Some evidence suggests ecallantide has a higher rate of hypersensitive reactions that further complicate its use [20].
Fresh frozen plasma (FFP) infusions have been used for ACEI-IA and are effective because they contain kininase 2 and ACE to directly breakdown bradykinin. Case series have reported the successful use of FFP in resolving angioedema symptoms. FFP may be a favorable agent in emergency use given its availability and relatively low cost; however, the risks for FFP infusion, including transfusion-related acute lung injury, fluid overload, and allergic reactions, pose a potential safety concern [32]. Additionally, there are some reports of worsening angioedema following FFP infusion, presumably due to initial spikes of kallikrein substrates [20,33]. In addition, there is a lack of large randomized controlled clinical trials that evaluate FFP use in the acute setting of ACEI-IA.
Purified C1-INH (such as Berinert) inactivates plasma kallikrein and factor XIIa to prevent downstream bradykinin and has been used in HAE, with several case reports supporting its use in ACEIA. C1-INHs have been shown to improve symptoms within 15 minutes of administration and up to 10.1 hours for complete resolution [8,21]. A case series reported that no patients receiving C1-INH required intubation, whereas five required intubation in the control group. Despite several successful case reports, no clinical trials are currently evaluating the use of C1-INH for ACEI-IA [33]. Its cost and lack of ready availability/access in the emergency department further limit its use.
CONCLUSION
Future randomized controlled trials of TXA use in ACEI-IA are needed to further support its efficacy and safety in this indication. There have been no direct comparator studies for ACEI-IA between different agents, and future studies will allow a better comparison of efficacy and adverse effects to optimize agent selection. Potential drawbacks to the use of TXA include the possibility of thrombotic events, adverse gastrointestinal effects, and a lack of large-scale studies that evaluate its use.
ACEI-IA remains a key unmet medical need, accounting for approximately 30% to 40% of angioedema emergency department visits [1]. As the ACEI class of medications is ubiquitously used for hypertension, heart failure, diabetes, and kidney disease, patients should be advised on the signs and symptoms of angioedema. Additionally, the gaining popularity of combination medications, the “polypill”, may further increase total ACEI exposure. Prompt treatment of ACEI-IA is needed to prevent intubation and improve clinical outcomes.
In this literature review, we highlight the valuable role of TXA in reducing intubation rates and improving angioedema symptoms in ACEI-IA. Notably, there were no deaths reported, and four patients requiring intubation in the evaluated studies were all intubated before TXA administration. TXA use may be advantageous due to availability, lower cost, and low prevalence of adverse effects compared to other off-label medications currently used for ACEI-IA.
Notes
Author contributions
Conceptualization: all authors; Investigation: GNP, TMT, AC; Resources: GNP, TMT; Supervision: BR, CM; Writing–original draft: GNP, TMT, AC; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
The authors received no financial support for this study.
Data availability
Data sharing is not applicable as no new data were created or analyzed in this study.
References
Article information Continued
Notes
Capsule Summary
What is already known
Angiotensin-converting enzyme-induced angioedema is primarily bradykinin-mediated with no treatments currently approved.
What is new in the current study
Tranexamic acid has the potential to resolve angioedema symptoms and prevent intubations.
