INTRODUCTION
Acute myocardial infarction (AMI) complicated by myocardial rupture is often fatal [1]. Myocardial rupture after AMI is generally reported as a subacute complication [2,3] and can occur in the early phase of AMI. If sufficient damage to the myocardium accumulates in the acute phase, myocardial rupture can occur in the early phase of AMI before or at emergency department (ED) arrival.
Clinical manifestations of myocardial rupture may differ based on the location of the injury and hemodynamic consequences. Myocardial rupture includes free wall rupture (FWR) and ventricular septal rupture (VSR), which may result in various significant hemodynamic derangements from the early phase of AMI. The incidence of myocardial rupture reported in clinical studies ranges from 0.31% to 0.45% [2ŌĆō4]. However, the incidence of myocardial rupture reported on the autopsy of myocardial infarction patients is up to 30.7%, which suggests that a large proportion of myocardial rupture remains undiagnosed [5]. Due to the high mortality rate following myocardial rupture, early diagnosis and prompt surgical treatment are crucial for survival. Therefore, emergency physicians (EPs) should be aware of myocardial rupture during emergency treatment of AMI.
Most current EDs are equipped with echocardiography for immediate performance in AMI patients with suspected myocardial rupture. However, only one report has been published on the early diagnosis of myocardial rupture using echocardiography performed by EPs [6]. The purpose of this study was to investigate the echocardiographic features of myocardial rupture detected by EPs using echocardiography in the ED.
METHODS
Ethics statements
This study was approved by the Institutional Review Board of Wonju Severance Christian Hospital (No. CR319132). The requirement for informed consent from patients was waived due to the retrospective nature of the study.
Study design and setting
This was a retrospective and observational study involving consecutive adult patients (>18 years of age) presenting with AMI in the ED of a tertiary care hospital from March 2008 to December 2019. The ED has approximately 46,000 annual patient visits and provides emergency care by residents and board-certified EPs. Emergency coronary angiography (CAG) and percutaneous coronary intervention are performed by cardiologists as needed throughout the day. A multifunctional ultrasonography machine, which can perform transthoracic echocardiography (TTE) and transesophageal echocardiography, is available 24 hours a day, 7 days a week in the ED. Emergency echocardiography is performed by EPs. Our training program for emergency echocardiography includes a 4-hour lecture, hands-on practice with an EP and cardiologist (SOH) for 1 month, and a minimum of 150 supervised examinations.
Selection of participants
Electronic medical records of the hospital were reviewed for cases of ŌĆ£MI and FWR or VSRŌĆØ based on the International Classification of Diseases, 10th Revision (ICD-10) code (I21, AMI; I51.0, acquired cardiac septal defect; I31.2, hemopericardium; I31.3, pericardial effusion; I31.9, disease of the pericardium; I23.0, hemopericardium as current complication following AMI; I23.2, ventricular septal defect as current complication following AMI; and I23.3, rupture of the cardiac wall without hemopericardium as current complication following AMI).
After reviewing the medical records and echocardiographic findings of these patients, those with myocardial rupture observed on echocardiography performed in the ED were included in the study. Patients whose myocardial rupture occurred during in-hospital stay, who had no echocardiographic evidence of myocardial rupture, or who had uninterpretable echocardiographic findings due to poor image quality were excluded.
Diagnosis and echocardiographic findings of myocardial rupture
When patients with suspected MI arrived at the ED, they received emergency cardiac care and underwent TTE performed by the attending EPs. Two ultrasound machines (Vivid E9, General Electric; EPIQ 7, Philips) equipped with 1 to 5 MHz phased array transducers were used in the ED during the study period. Our protocol of emergency echocardiography for patients with suspected AMI includes two-dimensional (2D) images from the parasternal, apical, and subcostal windows and color flow imaging. The initial echocardiographic diagnosis was made by EPs who performed TTE. Real-time consultation with another EP and cardiologist (SOH) was conducted if abnormal findings were observed on bedside echocardiography.
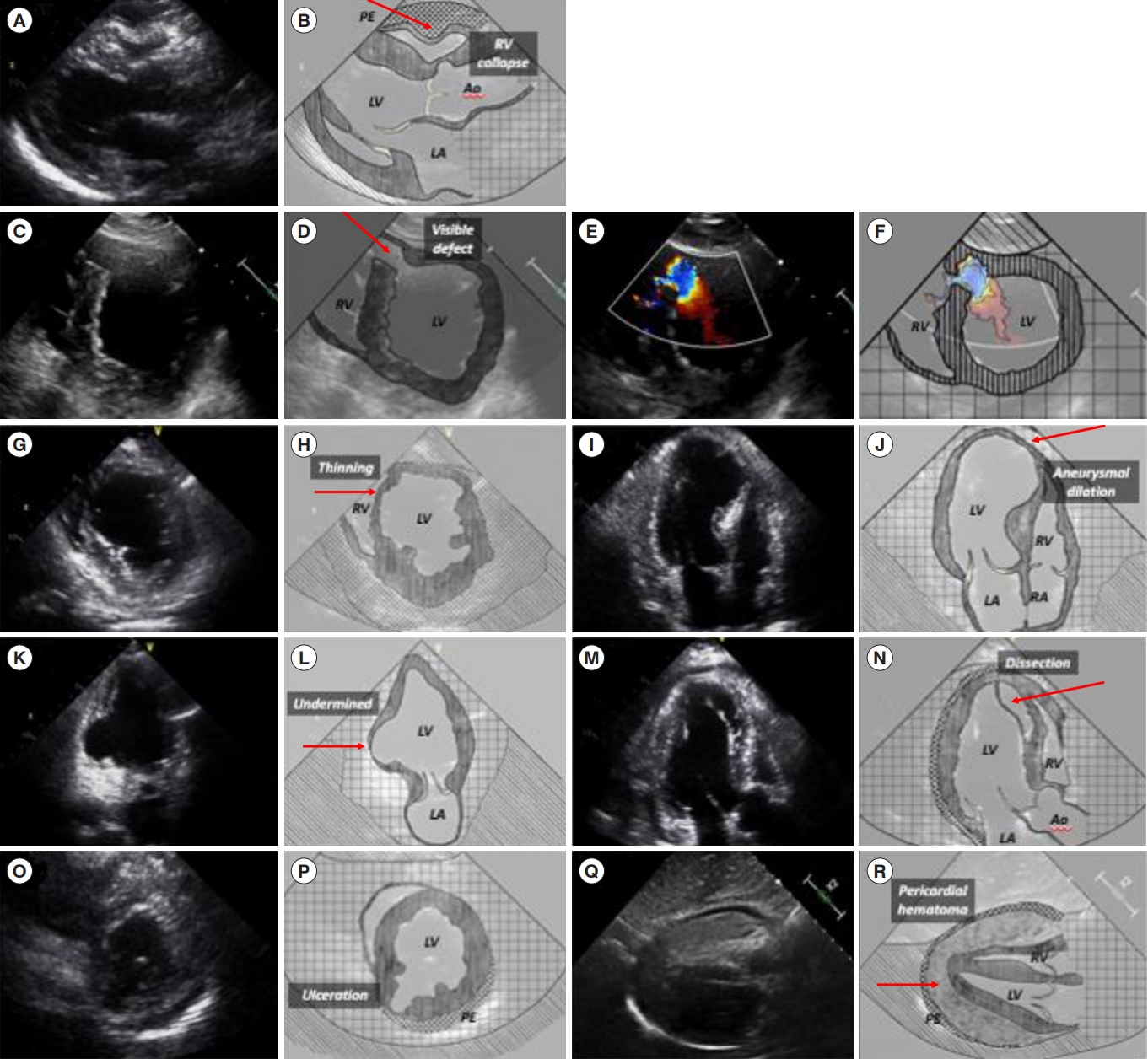
Echocardiographic findings were divided into diagnostic and additional features suggestive of myocardial rupture. Diagnostic features of myocardial rupture included a newly developed pericardial effusion for FWR or a visible shunt on the interventricular septum on 2D echocardiography or color flow imaging for VSR. Additional features suggestive of myocardial rupture were thinning or aneurysmal dilation of the affected myocardium; undermined myocardium such as endocardial disruption, ulceration, or dissection; abnormal wall motions such as fluttering or impingement; and pericardial hematoma. All acquired images were stored in a myocardial picture archiving and communication system and reviewed by an EP and cardiologist. The final diagnosis of myocardial rupture was made using comprehensive echocardiography by a cardiologist and/or surgical findings. Based on the echocardiographic findings, the type, features, and anatomical site of the rupture were investigated.
Clinical variables
The following clinical variables of patients were obtained from the medical records: age, sex, vital signs, chief complaint, comorbidities, smoking history, Killip classification at ED, area of MI, surgical operation, year of onset, time from chest pain onset to ED arrival, type of AMI, pulmonary edema on chest x-ray, left ventricular ejection fraction, serum troponin I level, cardiac arrest during a hospital stay, and in-hospital mortality.
Statistical analysis
Data were expressed based on the variable properties. Categorical data were reported as numbers with percentages for proportions and compared using Fisher exact test. Continuous data were reported as median (interquartile range) or mean┬▒standard deviation. Normality was assessed using the Shapiro-Wilk test. All statistical analyses were performed using IBM SPSS ver. 23.0 (IBM Corp), and the significance level was set at 0.05.
RESULTS
Patient characteristics
A total of 7,475 consecutive patients with AMI who presented to the ED was identified during the study period, and only 87 patients had corresponding diagnostic codes. Among the 87 patients, 62 were not compatible with the diagnosis, five had uninterpretable TTE findings due to poor image quality, and five developed myocardial rupture during the hospital stay and were excluded. Finally, 15 patients were included in the analysis (Supplementary Fig. 1, Supplementary Table 1).
The mean patient age was 73.7┬▒9.8 years, and nine patients (60.0%) were male. Hypertension was the most common comorbidity (seven patients, 46.7%), and only one patient (6.7%) had prior coronary artery disease. Three patients (20.0%) presented with AMI of Killip class IV. The anterior wall was the most common infarct location (53.3%). Four patients (26.7%) underwent surgery. The in-hospital mortality was 86.7% (Table 1).
Echocardiographic diagnosis of myocardial rupture by EPs
Myocardial rupture in 14 patients (93.3%) was diagnosed based on echocardiography performed by EPs. In one patient (6.7%), a diagnosis of VSR in the apical septum was missed. Types of myocardial rupture included FWR in eight patients (53.3%), VSR in five patients (33.3%), and combined rupture of FWR and VSR in two patients (13.3%) (Table 2).
Echocardiographic features of myocardial rupture
Diagnostic echocardiographic features were found in 100% of the patients with myocardial rupture, including pericardial effusion for FWR and a visible shunt on the interventricular septum for VSR. Additional echocardiographic features indicating myocardial rupture were thinning or aneurysmal dilatation in nine patients (60.0%), undermined myocardium in six patients (40.0%), abnormal regional wall motions in six patients (40.0%), and pericardial hematoma in six patients (40.0%) (Table 3, Fig. 1, Supplementary Table 2, Supplementary Video 1). The apical septal area was the most common site involved in myocardial rupture (Fig. 2).
DISCUSSION
In the present study, key echocardiographic features of myocardial rupture including pericardial effusion for FWR or visible shunt on the interventricular septum for VSR as well as various additional echocardiographic features suggestive of myocardial rupture were presented. The noninflammatory process is mostly attributed to myocardial rupture during the first 24 hours after AMI, although both inflammatory and noninflammatory processes, including cardiomyocyte apoptosis or defective myocardial remodeling, can be involved in the pathogenesis of myocardial rupture [7ŌĆō9]. Marked alteration of the skeletal framework and an acute change in connective tissue in the central zone of AMI may contribute to myocardial rupture [10]. The cardiac skeleton starts to collapse around the ischemic region within hours of myocardial injury from AMI.
Considering the possibility of myocardial rupture early after AMI, EPs should be aware of the potential presence of myocardial rupture when performing echocardiography in patients with AMI. For early diagnosis of myocardial rupture, echocardiography should be performed in the ED for all patients presenting with symptoms or signs of infarct extension [11]. However, EPs may miss myocardial ruptures if direct evidence is not observed because routine examination with color Doppler imaging focuses on valvular regurgitation. In the present study, diagnosis of myocardial rupture in the apical septum was missed on echocardiography. The EPs performed echocardiography, including routine examinations of color Doppler imaging on cardiac valves; however, they did not observe the affected area of myocardial rupture. This finding indicates that EPs should closely examine the possibility of mechanical complications when performing echocardiography in patients with AMI. In addition, highly prevalent areas of myocardial rupture including the anterior and inferior walls of the left ventricle, noted in the present as well as previous studies, should be closely observed during emergency echocardiographic examination [4,12,13]. In the present study, the incidence of FWR was higher than of VSR; however, in a study in which the mechanical complications during hospitalization were investigated, the incidence of VSR was higher than of FWR [14]. In the present study, the mechanical complications of myocardial infarction were investigated in the ED; in the previous studies, mechanical complications were investigated during the entire hospitalization period. The differences in incidence and pattern of mechanical complications are possibly due to the timing of identification of mechanical complications.
The present study provides detailed echocardiographic features of myocardial rupture. Key echocardiographic features of myocardial rupture were pericardial effusion for FWR and shunt flow through the interventricular septum for VSR. Additional echocardiographic findings suggestive of myocardial rupture may be accompanied by key echocardiographic features such as thinning or aneurysmal dilation of the affected myocardium; undermined myocardium, including endocardial disruption, ulceration, or dissection; and abnormal wall motions, including fluttering or impingement. Therefore, if additional echocardiographic features suggestive of myocardial rupture are observed, EPs should closely examine for myocardial rupture using color Doppler imaging to the affected area during emergency echocardiography.
The echocardiographic features observed in the present study are relatively consistent with the pathological classification of myocardial rupture, which is classified as follows. Type I is an abrupt tear in the wall without thinning; type II involves erosion of the infarcted myocardium covered by a thrombus; and type III is marked thinning of the myocardium and secondary formation of an aneurysm with a central perforation [15]. Type I myocardial rupture can be observed as a visible defect with shunt flow on echocardiography. Type II myocardial rupture can be observed as undermined myocardium including endocardial disruption or ulceration. Type III myocardial rupture is compatible with thinning or aneurysmal dilation with or without a shunt flow on echocardiography. However, autopsy findings may not match the echocardiographic features observed in vivo.
The present study has several limitations. It was a retrospective observational study conducted at a single institution with a relatively small sample size. Due to the nature of the study in which patients were retrospectively selected using ICD-10 codes, cases were possibly missed. Because the skills of an EP to perform echocardiography may vary by institution, limitations may exist in the general application of our experience to all emergency centers. Because only four of 15 patients underwent cardiac surgery due to death from in-hospital cardiac arrest or refractory shock, the relationship between the pattern of mechanical complications and prognosis was unknown. Because patients with mechanical complications undergo rapid deterioration of clinical status, the need for early diagnosis cannot be overemphasized. Autopsy on deceased patients could not be performed to confirm the diagnosis, preventing matching of the echocardiographic findings with pathological features of myocardial rupture.
In summary, the results of the present study showed that myocardial rupture, including FWR and VSR, can be observed with various findings on emergency echocardiography. EPs should be aware of the echocardiographic features suggestive of myocardial rupture for early diagnosis in the ED to help determine life-saving procedures such as pericardiocentesis or emergency cardiac surgery.