INTRODUCTION
Urolithiasis is one of the most common urological diseases worldwide, with a 1% to 13% prevalence depending upon region [1,2]. Urolithiasis commonly presents to the emergency department (ED) as renal colic with extreme pain. Millions of patients worldwide present to the ED annually with renal colic. The rate of renal colic presentations at EDs has been reported to be 6.7 to 27.9 per 1,000 ED visits [3]. Renal colic is a severe pain in the flank or abdomen, generally radiating to the groin or genital area, caused by obstructions in the urinary tract. The leading cause of renal colic is urinary flow obstruction and increased pressure proximal to the obstacle [1–3]. Although this pain is intense, most urinary tract stones are eliminated spontaneously and do not require surgical intervention. Therefore, effective analgesic treatment is a primary goal in managing renal colic [4–6].
Many therapeutic agents are prescribed for pain management in patients with renal colic, including opioid and nonopioid drugs. The commonly used nonopioid drugs are nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and α-blockers. NSAIDs and opioids are recommended as the first- and second-line treatments, respectively, under recent guidelines [4,7]. However, these drugs have many side effects and contraindications that require investigation of alternative and adjuvant drugs [7,8].
Ketamine is an anesthetic agent whose analgesic effects have recently been studied for renal colic. Studies have indicated intranasal and injectable ketamine to be effective in treating severe acute pain and renal colic, particularly compared with opioids [7,9]. The primary mechanism of action of ketamine is an antagonistic effect on the n-methyl-d-aspartate (NMDA) receptors. However, the analgesic effects have mainly been attributed to interactions with opioid receptors [10].
Desmopressin is another drug recommended for managing renal colic. Desmopressin is an analog of an antidiuretic hormone that has shown favorable effects with few side effects for treating renal colic in both sublingual and intranasal forms [4,5]. Renal colic results from acute dilatation of urinary tracts along with spasms of smooth muscles at the site of obstruction. This dilation leads to the release of prostaglandin E2, which causes diuresis by dilating the afferent arterioles and leads to further dilation of urinary tracts [11]. The marked antidiuretic effect of desmopressin is likely responsible for its efficacy in treating renal colic. Furthermore, desmopressin suppresses the contraction of the smooth muscle fibers of the renal pelvis, which might help pain management in renal colic. Likewise, stimulation of β-endorphin release can be effective in the analgesic effects of this drug; however, the mechanisms effective in relieving the pain of renal colic by this drug are still unknown [12,13].
Studies have indicated that patients prefer analgesics with immediate effects and painless administration routes. Common administration routes of analgesics, including oral, intravenous, and intramuscular, have limitations. For instance, oral administration is not common and routine for nothing by mouth patients. Intravenous injection requires the insertion of a peripheral venous catheter by emergency personnel. Intramuscular injection, in addition to pain at the injection site, is associated with delayed onset of drug action and is challenging in obese individuals. The intranasal administration route is favored due to its painlessness, fewer adverse effects, and sufficient effectiveness [9,14,15]. In the present clinical trial study, we investigated the analgesic effect of intranasal ketamine and desmopressin in renal colic patients referred to the ED.
METHODS
Ethics statement
This study was approved by the Research Ethics Committee of Isfahan University of Medical Sciences (No. IR.MUI.MED.REC.1400.191). The study protocol was registered in the Iranian Registry of Clinical Trial (No. IRCT20190422043340N12). Written informed consent was provided by all participants.
Study design and population
This double-blind, randomized clinical trial study was conducted in Alzahra and Kashani hospital EDs in Isfahan, Iran, from June 2021 to July 2022. Inclusion criteria included the presence of severe renal colic pain (visual analog scale (VAS) >5 in the flank or abdomen with or without radiating to the groin and genitalia) and a previous history of urolithiasis as diagnosed by an emergency physician. Accompanying symptoms and signs (i.e., dysuria, urine dribbling, and costovertebral angle tenderness) or laboratory findings supporting diagnosis such as hematuria may or may not have been present. Patient age was limited to 18 to 65 years. The diagnosis of renal colic was made based on history, physical examination, and urinalysis. After diagnosis and depending on the need, the presence of renal stones was confirmed in patients using ultrasound or computed tomography. Exclusion criteria were the following: having a history of hypertension, cardiac diseases, peptic ulcer or active gastrointestinal bleeding, chronic hepatic or renal failure, or any drug reaction; being pregnant or lactating; having unstable vital signs (systolic blood pressure <90 or >180 mmHg or heart rate <50 or >150 beats/min); receiving analgesics in the last 24 hours of hospitalization; loss of consciousness during the survey; and a final diagnosis of other than renal colic.
Randomization and blinding
Patients were randomly assigned to desmopressin, ketamine, or control groups using a computer-generated random number table with four blocks. Medications were prepared daily by an ED nurse based on patients' codes and labeled A (desmopressin), B (ketamine), or C (control); the labels were blinded to the researcher. The emergency physician who was blinded to the type of agents used a 1-mL syringe in each group to spray the prepared medication (0.5 mL in each nostril) using an intranasal Mucosal Atomization Device (Teleflex Medical). All patients were also blinded to their study group.
Interventions
Desmopressin group patients received intranasal desmopressin at a dose of 40 μg and intravenous ketorolac at 30 mg. In ketamine group patients, intranasal ketamine was administered at a dose of 1 mg/kg and intravenous ketorolac at 30 mg. Control group patients received an intranasal placebo (DB-SALINE 0.9%, DB Pharmacy) and intravenous ketorolac at a dose of 30 mg.
Study protocol
After completing ethics code preliminary requirements and training of emergency physicians, these emergency physicians selected eligible patients to participate in the study. The demographic information including age, sex, and body mass index was recorded. Patients were asked to determine their degree of pain using a VAS on a range of 0 to 10, in which 0 represents no pain and 10 represents the most intense [16]. Pain severity was recorded at baseline and at 10, 30, and 60 minutes after the beginning of the treatment. Vital signs, including heart rate, respiratory rate, and systolic and diastolic blood pressure, were recorded at baseline and at 60 minutes after the beginning of the study. The emergency physician regularly monitored patients during treatment. If the patient's pain was not reduced effectively (a 50% decrease in VAS score or attainment of a score ≤3) after 30 minutes of treatment, 0.1 mg/kg with a maximum dose of 5 mg of morphine was administered as the rescue analgesic and recorded. The primary outcome was the comparative reduction of VAS scores among the three groups after intervention. Secondary outcomes were the occurrence of hemodynamic changes and the need for rescue treatment.
Sample size
Assuming an α of 0.05 and β of 0.2, an 80% statistical power for the study, and final differences between the two test groups of at least 2 points on VAS [6], the necessary sample size was determined to be 40 in each group. To increase the power of study, 45 patients were included in each group.
Statistics analysis
Collected data were analyzed using IBM SPSS ver. 28 (IBM Corp). Frequency and percentage were used to describe qualitative data, and mean and standard deviation (SD) were used to describe quantitative data. Independent t-tests, chi-square tests, repeated measure analysis of variance (ANOVA), and one-way ANOVA were used for inferential analysis.
RESULTS
Enrollment included 135 patients (Fig. 1), their mean±SD age was 44.1±11.4 years, and 82 (60.7%) were men. All patients who were included in the study had a final diagnosis of renal colic and completed the study. Data from these patients were analyzed. Age, sex, weight, and morphine requirements are compared among the three treatment groups in Table 1.
As indicated in Table 1, the three groups did not have significant differences in age, sex, and weight (P≥0.05). Therefore, the demographic variables were not considered confounders. Also, the need for morphine was similar among the groups (P≥0.05).
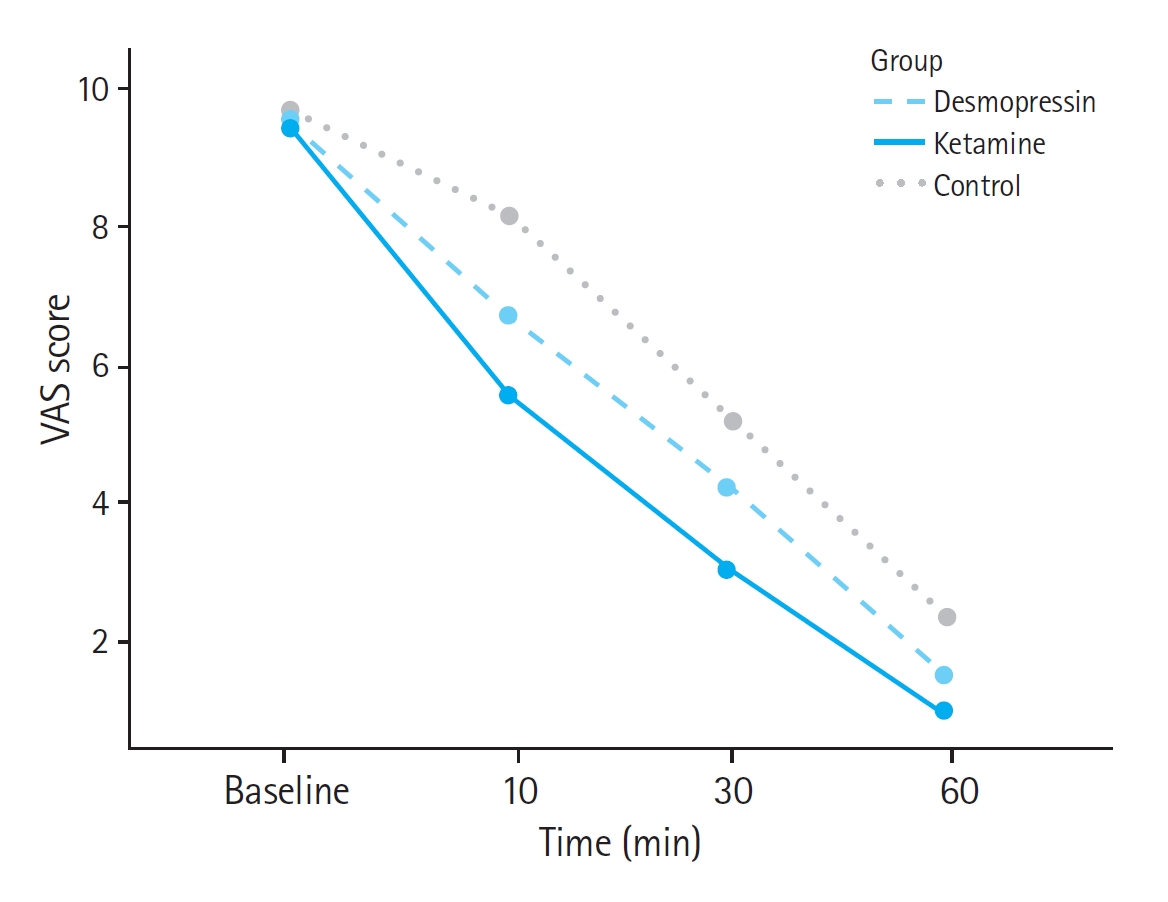
Table 2 indicates the mean±SD of the three groups' VAS scores during the treatment. The degree of perceived pain of the three groups at baseline was not significantly different (P≥0.05). Pain scores of the patients in the control group were higher than the other two groups at 10, 30, and 60 minutes after the intervention. Therefore, ketamine and desmopressin, along with ketorolac, were superior to ketorolac alone in relieving pain. VAS score differences between the ketamine and desmopressin groups compared to the control group were significant at 10, 30, and 60 minutes (P<0.05). Pain scores in the ketamine group at 10, 30, and 60 minutes were significantly lower than in compared to the desmopressin group (P<0.05).
DISCUSSION
Desmopressin is a synthetic replacement for antidiuretic hormone with more powerful and longer-lasting antidiuretic effects. This drug has advantages such as ease of administration and fewer contraindications and side effects than NSAIDs [17,18]. These anti-inflammatory agents can lead to acute kidney failure (AKI) by reducing the glomerular filtration rate (GFR) and renal blood flow in a kidney that is already at risk of failure due to hydronephrosis. Studies have shown that desmopressin exerts its diuretic effects without affecting renal blood flow or GFR. Significant adverse effects of desmopressin, such as hypotension, tachycardia, hyponatremia, and gastrointestinal symptoms, usually resolve within 24 hours after administration and are more noticeable in older patients or with repeated administrations. Our study showed that the patients who received desmopressin did not have a significant difference in vital signs compared to other treatment group patients 1 hour after treatment.
Some human studies investigating desmopressin's effectiveness on renal colic have had conflicting results. However, most previous studies examined the effects of desmopressin in the short term and were limited to hospitalized patients in the ED [17–19]. In our study, patients receiving desmopressin and ketorolac treatment reported lower pain scores 10, 30, and 60 minutes after the treatment compared to ketorolac alone. Hence, our study confirms the immediate effects of desmopressin in renal colic patients, which is consistent with previous studies [4,5,19]. Arhami Dolatabadi et al. [4] aimed to investigate the effectiveness of intranasal desmopressin on renal colic patients compared to intravenous ketorolac. The pain of the patients who received only desmopressin decreased significantly in the first 10 minutes, as well as the pain of those who received diclofenac. Until the 20th minute, the desmopressin group patients' pain scores decreased but then tended to rise. Also, during the study, there was no significant difference in the pain scores of patients receiving diclofenac alone and of those receiving diclofenac along with desmopressin. However, at the 30th minute, the VAS scores of patients receiving combination therapy were insignificantly lower. Furthermore, fewer patients receiving combination therapy reported that their pain intensity had not changed [20]. Consistent with Lopes et al. [20], our patients in the desmopressin group had a lower morphine requirement than the controls, though this difference was not statistically significant. While some previous studies concluded that treatment with desmopressin alone had no favorable results [21], we concluded that in combination with NSAIDs and primarily in the first minutes of treatment, desmopressin can increase the effectiveness of the treatment and, as a result, reduce the dosage of prescribed NSAIDs. The dosage of desmopressin in this and most previous studies was important, 40 μg [17,19]. Pricop et al. [11] showed that doses of 60 and 120 μg of sublingual desmopressin have equal or greater efficacy than NSAIDs in renal colic patients.
Ketamine is an NMDA receptor antagonist widely used for anesthesia. In recent decades, low-dose ketamine has been used to treat moderate to severe pain [16]. The analgesic mechanisms of this drug are not limited to interactions with NMDA receptors. Studies have shown that ketamine has agonistic effects on opioid receptors, γ-aminobutyric acid receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, and cholinergic and dopaminergic receptors. However, the analgesic mechanism of this drug is unknown [10]. In recent years, the effectiveness of this drug on renal colic patients has been examined [9,22–24]. The study of Hosseininejad et al. [7] investigated and compared the effects of intravenous ketamine and morphine on renal colic. The results of this study indicated that the combined treatment of morphine and ketamine was significantly more effective than morphine alone; However, patients receiving ketamine demonstrated more adverse effects and changes in vital signs. In the present study, the vital signs in patients receiving ketamine were not significantly different from other patients; this can be attributed to the intranasal route of administration of ketamine in our study.
Although intravenous ketamine has shown promising analgesic effects, the analgesic effects of intranasal ketamine have been studied in recent years; ketamine has shown favorable effectiveness and few adverse effects in this case [9,14]. The intranasal form of ketamine reaches a detectable concentration in the blood after 2 minutes and reaches its maximum effects within 30 minutes, which is one of the factors that make this drug suitable for managing renal colic patients [24]. Our study showed that patients receiving intranasal ketamine with intravenous ketorolac had significantly less pain severity than those receiving ketorolac alone or combined with desmopressin throughout the study. Therefore, unlike desmopressin, ketamine has shown favorable results in causing a fast and stable analgesic response. Sotoodehnia et al. [25] investigated and compared the effect of intravenous ketamine to that of ketorolac in relieving pain in patients with renal colic. There was no significant difference in the mean pain scores of the two groups of patients during the study. Similar results were observed in the study of Khavanin et al. [9] in which the mean pain scores of the patients in the intranasal ketamine group were significantly lower in the 5th minute posttreatment. Also, in the study, hospitalization duration and the need for additional analgesics were significantly lower in the ketamine group; patients were significantly more satisfied with their pain relief. In addition, the two groups did not differ significantly in terms of vital signs during the study [9]. Pouraghaei et al. [22] found intranasal ketamine to be as effective as intravenous morphine for pain control in renal colic.
The present study had limitations. This study was a single center study due to the limited budget. In addition to not recording and investigating non–life-threatening adverse effects of treatments, this study examined the patients only for one hour and can provide no information on long-term outcomes and possible side effects. Also, the changes in VAS cannot be explained by the effects of the study drugs alone because the sample size was small and the effect of ketorolac cannot be ignored. In the control group, patients receiving ketorolac and a placebo also had a significant improvement in their level of pain. To eliminate the effect of ketorolac in future studies, we recommend that ketamine and desmopressin be used as the only drugs.
Despite the limitations, the present study had strengths. Among these strengths was the large sample size and the longer follow-up period compared to previous studies, strengthening our study's results. Because the ideal analgesic doses of ketamine and desmopressin are not known, we recommend the conduct of multicenter studies with larger sample sizes and longer follow-up duration.
Ketamine had favorable analgesic effects in renal colic patients. Intranasal ketamine had better pain control as compared to intranasal desmopressin, although desmopressin showed efficacy in the first minutes after treatment. The need for rescue treatment in the ketamine group was less than in other groups, but this finding was not statistically significant.