INTRODUCTION
Rapid airway management is a critical component of care in the acutely ill and decompensating patient. National data suggest that there are nearly 400,000 intubations performed in the emergency department (ED) each year [1]. These intubations are at increased risk of complications due to their emergent nature [2]. One large multicenter trial of intubations among the critically ill [3] reported severe complications (i.e., hypoxia, hypotension, or cardiac arrest) in 18% of patients. Another ED-based study [4] reported a 12% adverse event rate, including events such as esophageal or mainstem intubation, hypoxia, and cardiac arrest. These event rates are likely even higher among patients with difficult airways or those in cardiac arrest [2].
The use of point-of-care ultrasound (POCUS) for emergency procedures to supplement or transform conventional techniques is increasing rapidly [5–9]. Therefore, it is not surprising that this has also extended to airway management [10,11]. Ultrasound can be used to assess for a difficult airway prior to intubation, allowing time for appropriate preparation and secondary plans for airway management. Additionally, POCUS can be used to confirm proper placement and depth of the tube. After intubation, ultrasound can be utilized to assess and identify problems preventing adequate ventilation. In the event of a known difficult intubation, POCUS can be leveraged to accurately identify the cricothyroid membrane even when external anatomy is challenging for palpation.
In this article, we will outline the application of ultrasound for airway assessment, confirmation, and management as well as provide guidelines for troubleshooting a decompensating ventilated patient.
ASSESSMENT OF THE DIFFICULT AIRWAY
Up to 90% of difficult airways are unanticipated [12]. The management of the difficult airway presents a significant challenge in clinical practice, and as such, various tools and techniques have been developed to help make accurate assessments prior to intubation. Traditionally, this has been done using risk factors and physical examination maneuvers. Numerous assessment tools have been reported with variable diagnostic accuracy [13]. The upper lip bite test is often considered to have the best diagnostic accuracy, though one study found that the sensitivity was only 67% [14–16]. Another commonly used tool, the modified Mallampati classification, had a sensitivity of 53% and a specificity of 89% [16].
Having the ability to assess the airway prior to intubation is a valuable skill and one that should be done quickly and accurately. POCUS is a helpful adjunct in airway assessments and can be done rapidly and noninvasively. There are several key measurements that can be used to predict difficult airways.
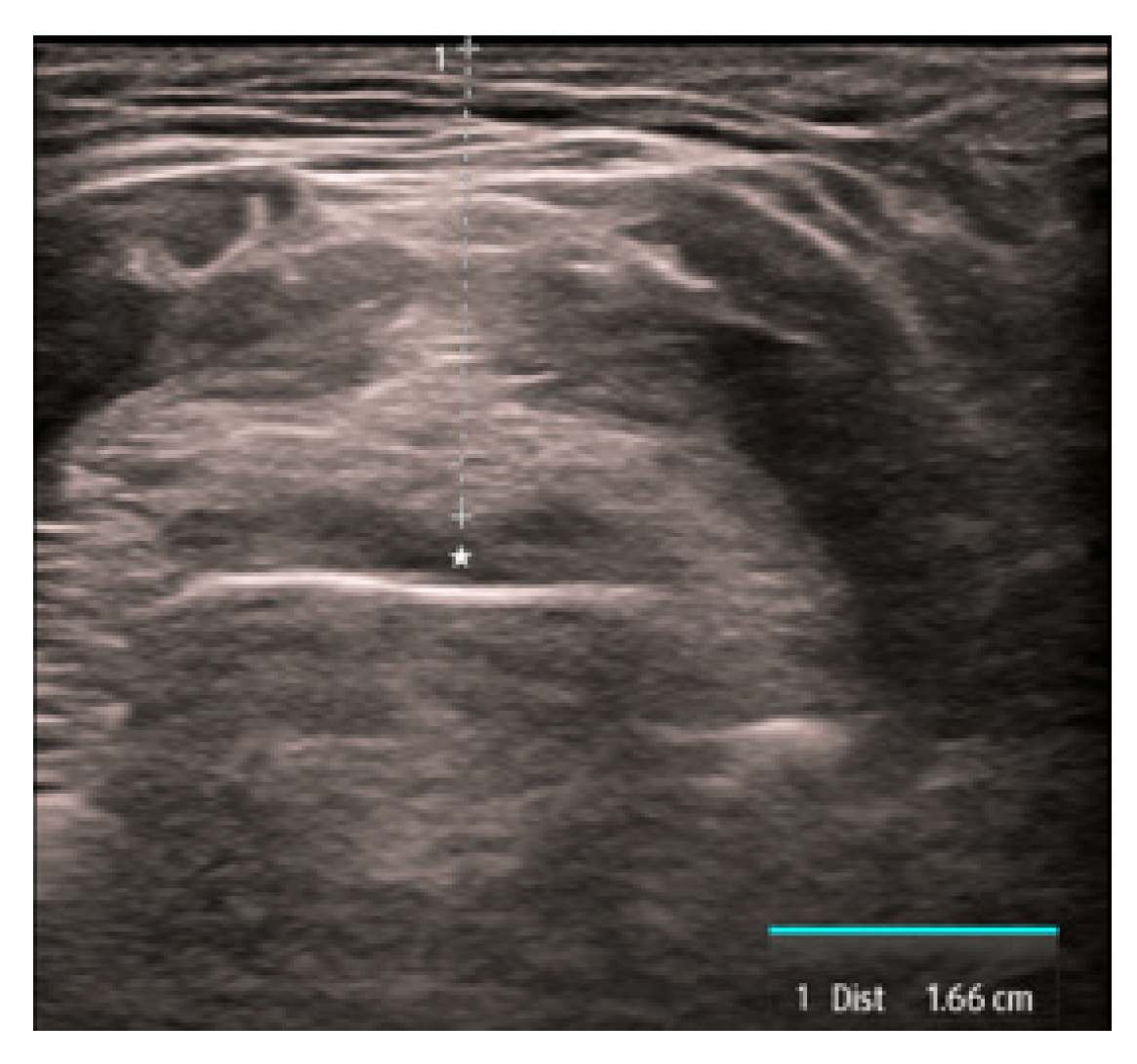
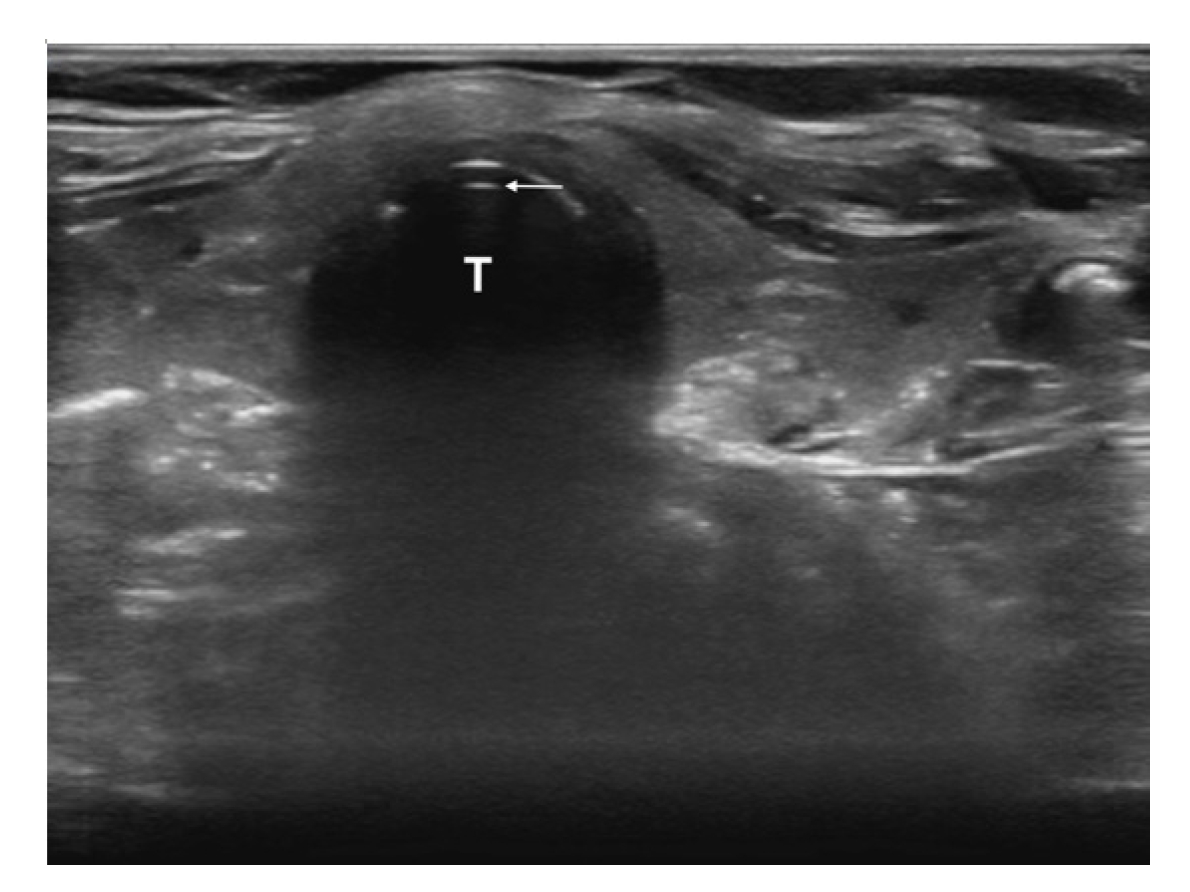
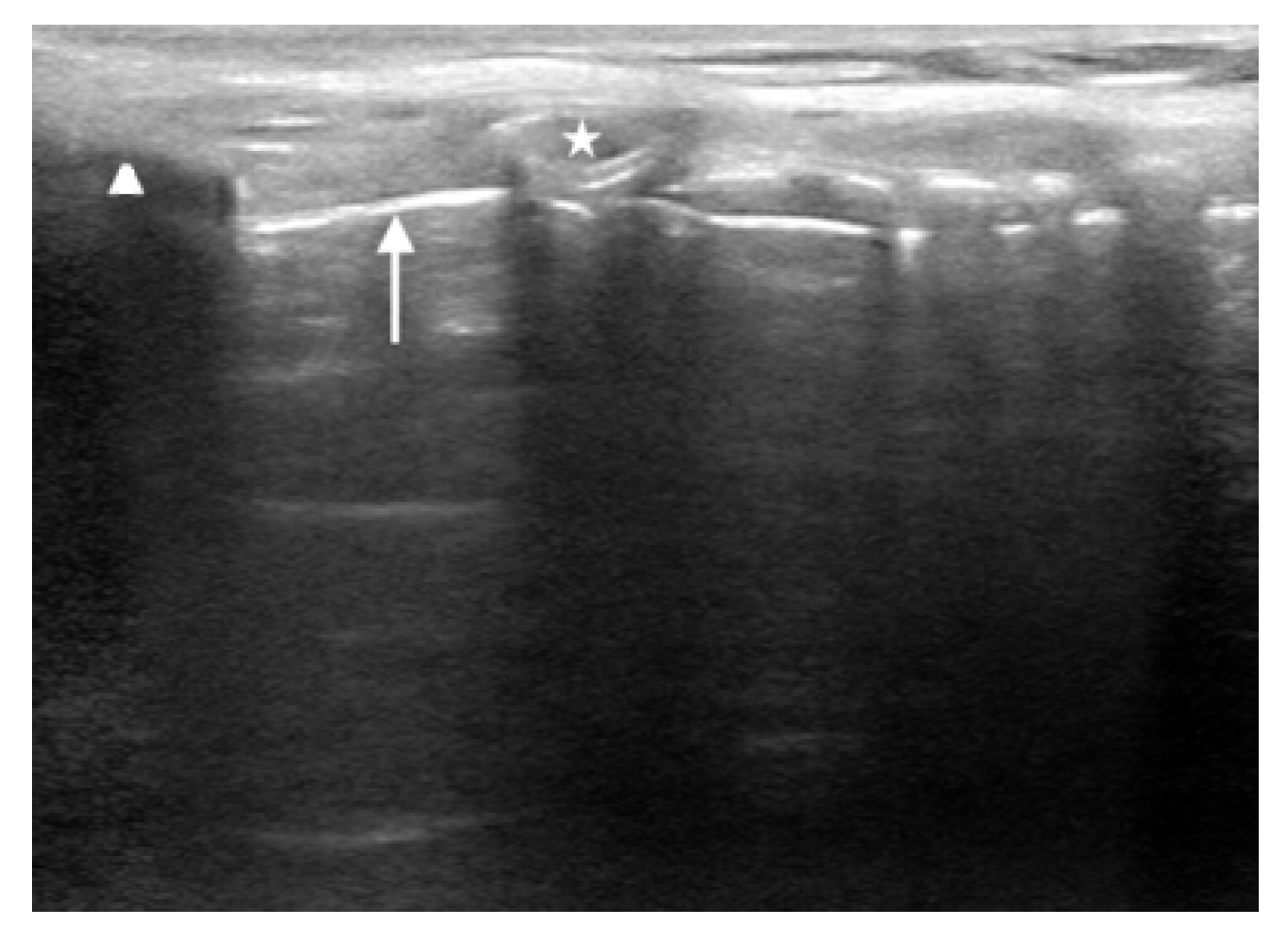
The first is the distance from the skin to the epiglottis (DSE) [17–22]. This measurement is taken at the level of the thyrohyoid membrane. The linear transducer is placed transversely in the midline just superior to the thyroid cartilage. The epiglottis is seen just deep to the thyrohyoid membrane and preepiglottic space and will appear as a hypoechoic linear structure above the air-mucosa interface (Fig. 1). The distance is then taken from the superficial skin edge to the anterior edge of the epiglottis. In a recent systematic review and meta-analysis [22], a DSE of ≥2.54cm had a sensitivity of 82% and a specificity of 91% for predicting a difficult airway.
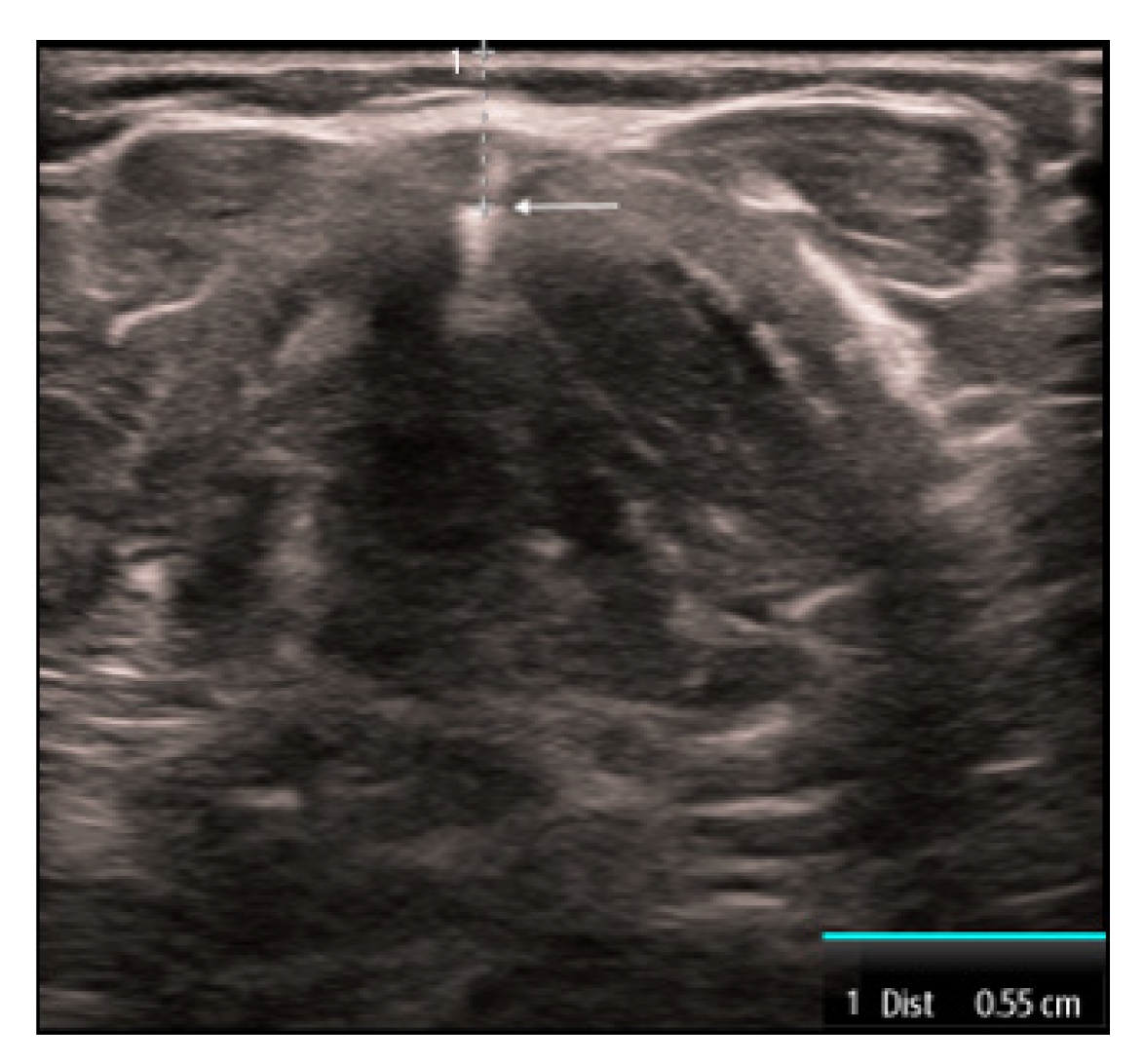
The second measurement is the distance from the skin to vocal cord (DSVC). This measurement is obtained by placing the linear transducer transversely in the midline over the thyroid cartilage. The strap muscles are seen anteriorly to the thyroid cartilage and the vocal cords are visualized just deep to the thyroid cartilage. The measurement is taken from the skin to the anterior commissure where the vocal cords join together (Fig. 2). A recent systematic review and meta-analysis [22] found that the DSVC had an overall sensitivity of 75% and a specificity of 72%. However, the literature is controversial with variable thresholds used and some even reporting an inverse association [23,24]. Therefore, the utility of this measure remains limited.
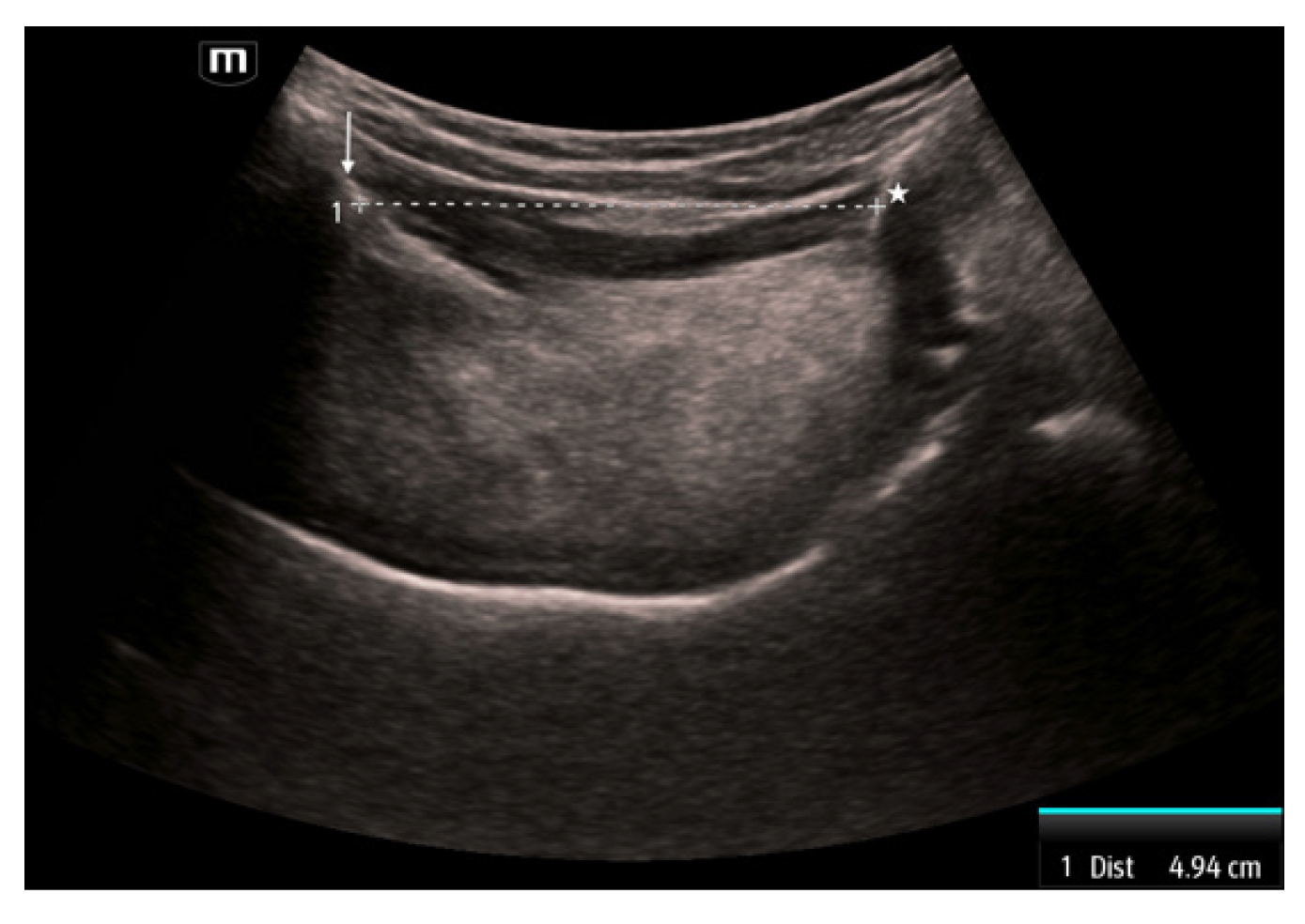
The third measurement is the hyomental distance (HMD), which is measured between the mentum of the mandible and the hyoid bone [21]. This measurement is obtained by placing the curvilinear transducer sagittally in the midline with the superior portion abutting the mandible. The mentum is seen as a hyperechoic structure with shadowing behind it on the superior portion of the image. The hyoid is a hyperechoic structure with shadowing behind it in the inferior portion. The measurement is taken between these two structures (Fig. 3). This distance has been reported as a single HMD, as a ratio of the HMD measured with the head in a ramped position compared to the head in neutral position (HMDR1), and the ratio of the HMD measured with the head in maximal extension compared to the head in neutral position (HMDR2) [21]. Patients with a shorter HMD in neutral position (<4.0 cm) were more likely to have a difficult airway [25]. Using a cutoff of 5.29 cm, HMD in the neutral position was 96.7% sensitive and 71.6% specific [26]. HMDR1 has less diagnostic utility with a 75% sensitivity and 76.2% specificity when using a threshold of 1.12 [27]. In contrast, HMDR2 has been reported to be 100% sensitive and 90.5% specific when using a threshold of 1.23 [27].
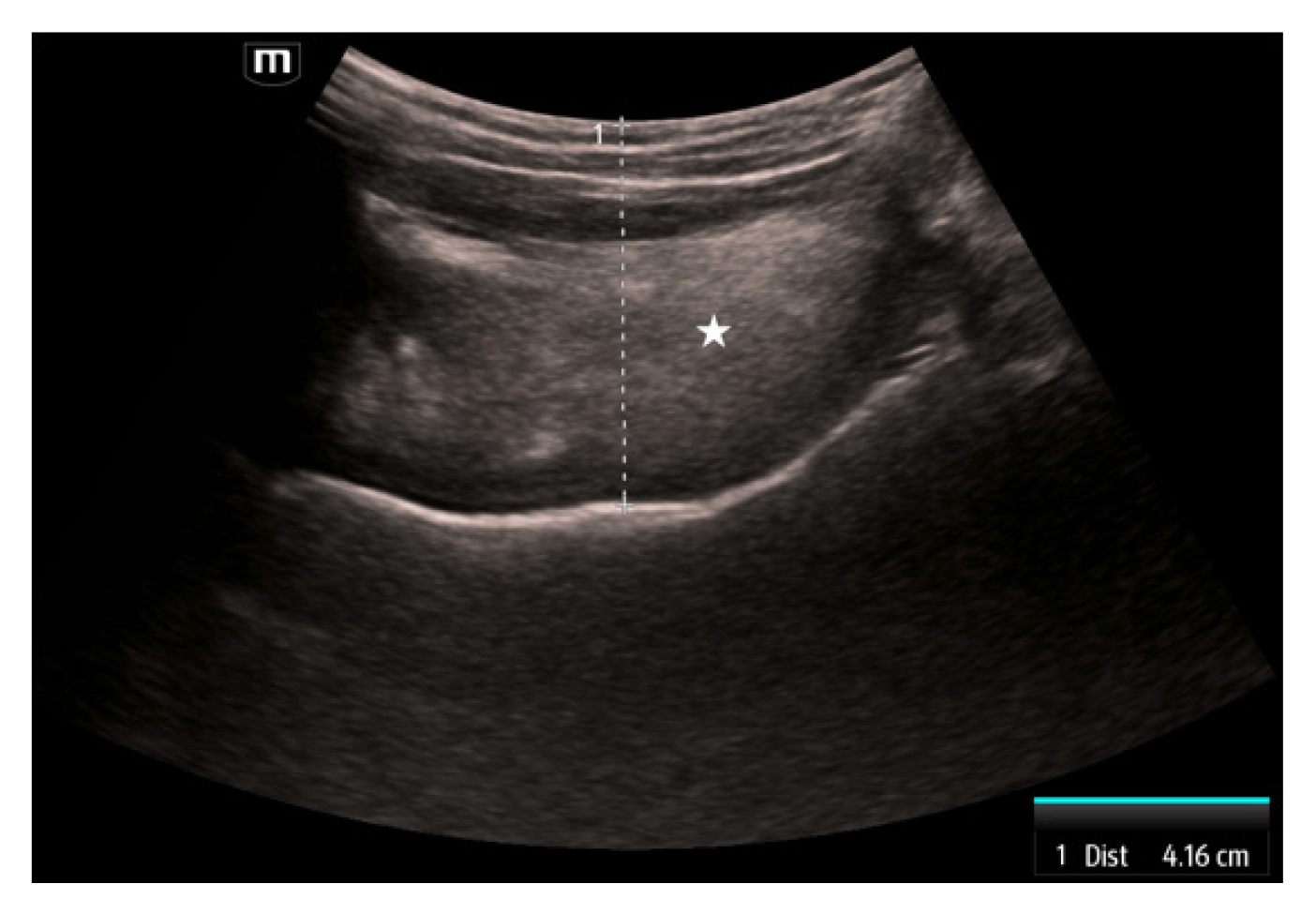
The fourth measurement is the tongue thickness. The tongue is visualized using a linear transducer placed transversely underneath the chin. The tongue is measured in the midline at the widest diameter in a superficial to deep direction beginning at the skin (Fig. 4). Tongue thickness has been demonstrated to be greater in difficult versus easy laryngoscopy (6.1–6.2 cm vs. 5.3–5.8 cm) [28,29]. When using a threshold of 6.1 cm, tongue thickness is 71% to 75% sensitive and 72% specific [28,29].
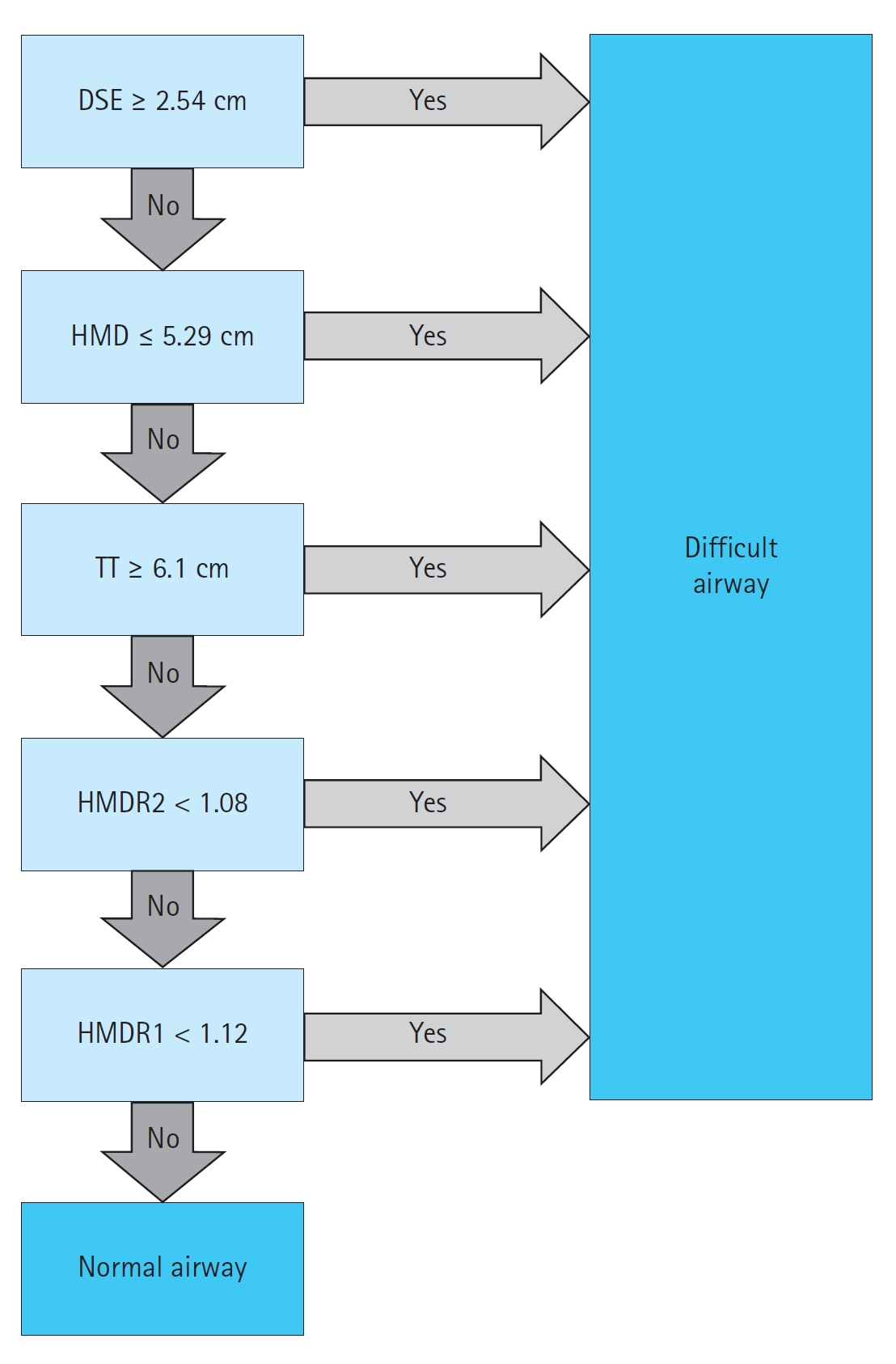
Lin et al. [21] proposed a protocol for this assessment, the Difficult Airway Evaluation with Sonography (DARES). The DARES protocol uses DSE, tongue thickness, HMD, HMDR1, and HMDR2 to predict a difficult airway (Fig. 5). An airway is considered difficult if any of the findings are positive. While informed by existing literature, this algorithm has not been externally validated.
INTUBATION CONFIRMATION
After the intubation is performed, it is critical to confirm that the endotracheal tube (ETT) was correctly placed in the trachea. Data suggest that the first-pass success rate for endotracheal intubation is 84% in the ED and 78% in the prehospital environment [30,31]. Confirmation of ETT location typically involves direct visualization of the ETT passing through the vocal cords followed by one or more confirmatory techniques. Auscultation of bilateral breath sounds, visualizing misting of the ETT, or using an esophageal detector device have limited diagnostic accuracy for confirming ETT placement [11,32–37].
While waveform capnography is more accurate than the aforementioned approaches, it can be limited by false positives in the case of hypopharyngeal placement or recent ingestion of a carbonated beverage, as well as false negatives when expired CO2 levels are low (e.g., flash pulmonary edema, massive pulmonary embolism, or cardiac arrest) [11,20,38,39]. Importantly, data suggest that the accuracy of end-tidal capnography in cardiac arrest may be as low as 64% [33–35]. Capnography also requires several positive pressure ventilatory attempts, which can increase gastric distension and increase the risk for aspiration.
In contrast, POCUS can allow rapid confirmation during or after the intubation attempt with a relatively short training period [10,40]. Among adults, POCUS is 99% sensitive and 97% specific [10,41]; whereas, POCUS is 92% to 100% sensitive and 100% specific in pediatric patients [42]. Studies have reported consistent accuracy regardless of the ETT size or type of transducer (e.g., linear, curvilinear) used [43,44].
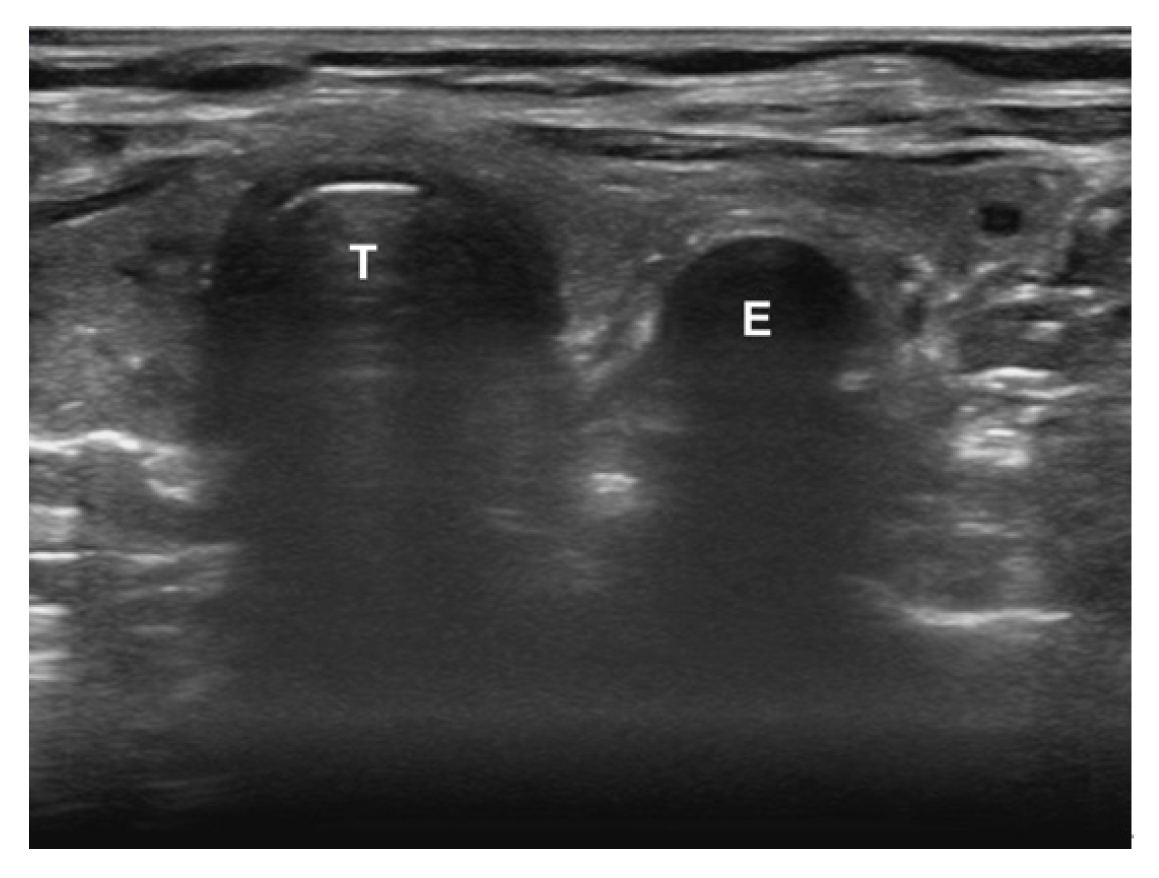
To confirm the ETT location, begin by placing the ultrasound transducer across the trachea in a transverse orientation. The ideal location is just superior to the suprasternal notch, as this has been shown to have superior visualization and diagnostic accuracy compared with other locations [45,46]. Confirmation can be performed in real-time (i.e., dynamic) or after the intubation (i.e., static). With the dynamic technique, the sonographer is assessing for rapid flutter-like movement as the ETT passes through the vocal cords (often referred to as the “snowstorm sign”) [20]. The static technique assesses for the appearance of either a thin hyperechoic membrane just posterior to the trachea (i.e., tracheal intubation) (Fig. 6) or a second air-mucosa interface referred to as the “double tract” sign (i.e., esophageal intubation) (Fig. 7) [20].
The literature has not demonstrated a significant difference in the accuracy between the static and dynamic techniques, so either are acceptable [10,47]. However, the static technique offers several unique benefits, including only requiring a single clinician (i.e., the intubator can also perform the POCUS immediately after the intubation is performed) and not placing the transducer on the neck during the intubation, which could make the intubation more challenging [20]. The major disadvantage is that it can be more difficult to locate the ETT behind the trachea due to the air artifact. In fact, data have shown reduced confidence and longer time to identification when the ETT is endotracheal versus esophageal [48]. To address this, some authors recommend twisting the ETT between one’s fingers to induce a false motion artifact [49,50]. While there was no significant difference in overall accuracy, ETT twisting has been shown to improve confidence and time to ETT identification [49]. Others have proposed infusing the ETT cuff with saline instead of air to better visualize the cuff [51–53] .
In addition to direct visualization with transtracheal ultrasound, indirect signs such as lung sliding or diaphragmatic excursion can also be used to confirm ETT location. Bilateral lung sliding has been reported to be 92% to 100% sensitive and 56% to 100% specific [54–59]. Similarly, diaphragmatic motion has 91% to 100% sensitivity and 50% to 100% specificity [60–62]. When combined with transtracheal ultrasound, several studies [56,57] have found that the addition of lung sliding improves the accuracy compared with transtracheal ultrasound in isolation.
ASSESSMENT OF ETT DEPTH AND UNILATERAL LUNG INTUBATION
After confirming that the ETT is within the trachea, the next step is to assess the depth. If the ETT is placed too shallow, it can cause damage to the vocal cords or become dislodged [63–65]. If the ETT is placed too deep it risks barotrauma in the ventilated lung along with atelectasis and hypoxia in the unventilated lung [65,66]. Data suggest that incorrect ETT depth can occur in up to 15% of adults and 18% of children [67,68].
Clinical markers like chest rise and breath sounds have limited accuracy for mainstem intubation and cannot provide any insight into the ETT depth [69–71]. While chest radiography is considered the gold standard for ETT depth, it can take a significant amount of time, exposes the patient to radiation, and may delay other aspects of care in a critically ill patient [72].
In contrast, POCUS can provide rapid assessment of ETT positioning and assess for signs of mainstem intubation. In adults, transtracheal ultrasound had 84.8% accuracy for determining ETT depth and took on average 19 seconds to perform [73]. A recent systematic review and meta-analysis [74] found that ultrasound had 86.7% accuracy for detecting mainstem intubations, with a sensitivity of 93.0% and a specificity of 75.0%. In pediatric patients, one study [75] used a three-point technique (transtracheal ultrasound plus bilateral lung sliding) and reported 85.7% sensitivity and 98.3% specificity for mainstem intubation. Another study [76] used a saline-filled ETT cuff and was able to correctly identify ETT depth in 95% of cases.
To assess the depth of the ETT, start by placing the ultrasound transducer just superior to the suprasternal notch in the sagittal plane. Identify the thyroid cartilage, cricoid cartilage, and tracheal rings as hyperechoic, rounded structures in the near field with gray shadows. The ETT cuff will appear as a second, hyperechoic line directly posterior to these structures (Fig. 8). Saline can be instilled to improve visualization of the ETT cuff. If the cephalad border of the ETT cuff is seen at or above, the first tracheal ring it is considered too high; between the second and eighth tracheal rings is considered adequate; and below the eighth ring or not visualized is considered too deep [73]. One additional benefit of POCUS is real-time assessment. If the ETT is visualized either above or below the ideal location, it can be advanced or retracted using real-time guidance.
If there is any concern for mainstem intubation or the ETT cuff cannot be adequately visualized, the transducer should be placed longitudinally on the anterior chest at the midclavicular line to assess for lung sliding bilaterally [54–59,75]. Unilateral lung sliding may suggest a mainstem intubation but can also be seen in pneumothorax or when large blebs are present [20]. To distinguish a mainstem intubation from other causes, one can assess for the presence of a lung pulse [65,77,78]. Lung pulse refers to the visualization of the rhythmic movement of the visceral pleura against the parietal pleura resulting from cardiac pulsations through an airless and motionless left lung. Lung pulse is 93% sensitive and 100% specific for right mainstem intubation [77].
IDENTIFYING THE CRICOTHYROID MEMBRANE
Cricothyrotomy is a critical procedure in scenarios where intubation and ventilation are unsuccessful. While uncommon, this is a high-risk procedure with increased risk of poor outcomes [79–81]. This procedure is fraught with complications stemming from anatomic challenges, high-stress conditions, and limited opportunities to practice the technique in a controlled, high-fidelity setting [82–84]. One study [85] found that only 36% of anesthesiologists were able to successful perform a cricothyrotomy in practice using landmark technique. Another study [86] found that among nonobese women, anesthesiologists could accurately identify the cricothyroid membrane (CTM) in 71% of cases, whereas that declined to 39% among obese patients.
Ultrasound can allow for direct visualization of the CTM, as well as any complicated anatomy, such as masses, enlarged thyroid glands, or vascular structures which would complicate an open cricothyrotomy. Ultrasound substantially improves the accuracy of CTM identification, with one study [87] reporting a twofold increase in successful identification in average patients. Among those with difficult or poorly defined anatomy, the accuracy improves by fivefold to tenfold [88,89].
Studies suggest the technique is both feasible and has a quick learning curve. Clinicians have shown to be able to identify the CTM membrane confidently and competently in both simulated and live patients [83,90,91]. Among clinicians without prior airway ultrasound experience, this has been shown to remain accurate with minimal training and has a high retention rate [84,92,93]. Moreover, this can be performed in less than 30 seconds across multiple patient populations, including pediatric and obese patients, and takes a comparable amount of time compared to the landmark-based approach [83,94,95].
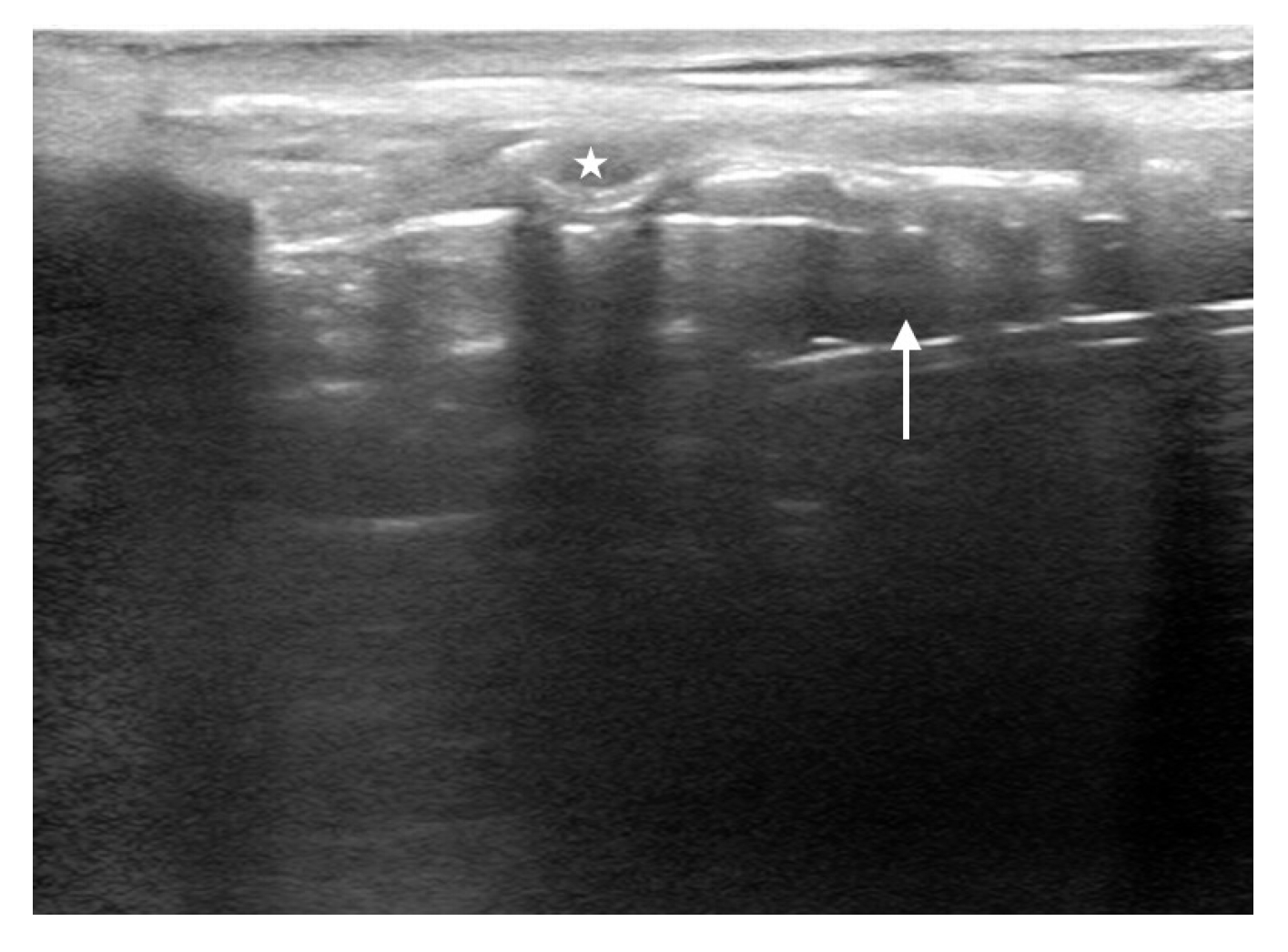
The technique to identify the CTM is similar to ETT depth determination described above. Lay the patient supine with their neck extended. Start with the transducer in the sagittal plane on the anterior neck just inferior to the thyroid cartilage. Identify the tracheal rings, which will appear as a “string of pearls” leading from caudal to cranial direction. Immediately superior to that will be a larger ring (i.e., cricoid cartilage), followed by a hyperechoic membrane and then a larger rectangular structure (i.e., thyroid cartilage). The hyperechoic line between and just deep to the cricoid and thyroid cartilages is the CTM (Fig. 9). Slide a blunt needle under the cranial portion of the transducer down to the corresponding location of the CTM and subsequently mark the skin with a surgical pen [84].
Best practice is to mark the CTM prior to the intubation, rather than attempting to identify it after a failed intubation attempt. The CTM should be marked in the position that the cricothyrotomy would be performed. One study [96] found that the midpoint of the CTM moved caudally an average of 4.2 mm when changing the head of bed elevation from 90° to 0° and this effect was increased if the patient was obese or over age 70 years [96]. Moreover, when the CTM is marked in the neutral neck position, the marking can move outside the CTM border when the neck is converted to full extension [97,98]. Fortunately, the CTM will return to the correct location when the patient is returned to the same position as when they were originally marked [99]. Therefore, it is advisable to mark the CTM in neck extension as close to the anticipated procedural position as possible prior to any attempted airway intervention [84,100].
ULTRASOUND FOR THE CRASHING VENTILATED PATIENT
Ultrasound also offers benefits in the assessment of the ventilated patient who experiences clinical deterioration. Traditionally, assessment of the postintubation patient has relied primarily on indirect assessment using structured algorithms, such as DOPES (dislodgement, obstruction, pneumothorax, equipment malfunction, stacking of breaths). However, as POCUS has expanded, we propose an update algorithm: Sono-DOPES. This algorithm builds upon previous work in DOPES (which had relied upon primarily physical examination findings) and adds ultrasound to enhance each stage (Table 1).
CONCLUSION
Airway management is a common procedure within emergency and critical care medicine. Traditional techniques for predicting and managing a difficult airway each have important limitations. As the field has evolved, POCUS has been increasingly utilized for this application. Ultrasound can be utilized to predict difficult airways, confirm ETT location and depth, locate the CTM, and facilitate the assessment and management of the crashing patient on mechanical ventilation.