Dear Editor,
Carbon monoxide (CO) poisoning is the leading cause of pediatric poisoning mortality in the United States—most frequently resulting from house fires or unintentional indoor CO exposures—and leads to about 5,000 pediatric emergency department visits annually [1–3]. In pregnancy, the fetus is particularly susceptible to CO [4]. Fetal hemoglobin has a higher affinity than adult hemoglobin for both oxygen and carboxyhemoglobin (COHb). Fetal COHb concentration may be about 15% higher in the fetus than in the mother [5], but simultaneous measurements in vivo are rare. Longo and Hill [6] concluded that to normalize fetal COHb, a pregnant woman would need to continue oxygen therapy five times longer than it took for her own COHb to normalize. We describe a case of antepartum CO poisoning at term, treated with caesarean section and normobaric oxygen with measurement of maternal and fetal COHb within 2 hours of one another.
A 21-year-old woman (who has been pregnant twice and given birth once) suffered acute unintentional CO poisoning at estimated gestational age of 38 weeks and 5 days. She had adequate prenatal care, normal prenatal screenings, and a positive Group B Streptococcal culture. Her only regular medication was a prenatal vitamin and she reported intermittent cannabis use. She consented to sharing anonymized clinical details for herself and her baby.
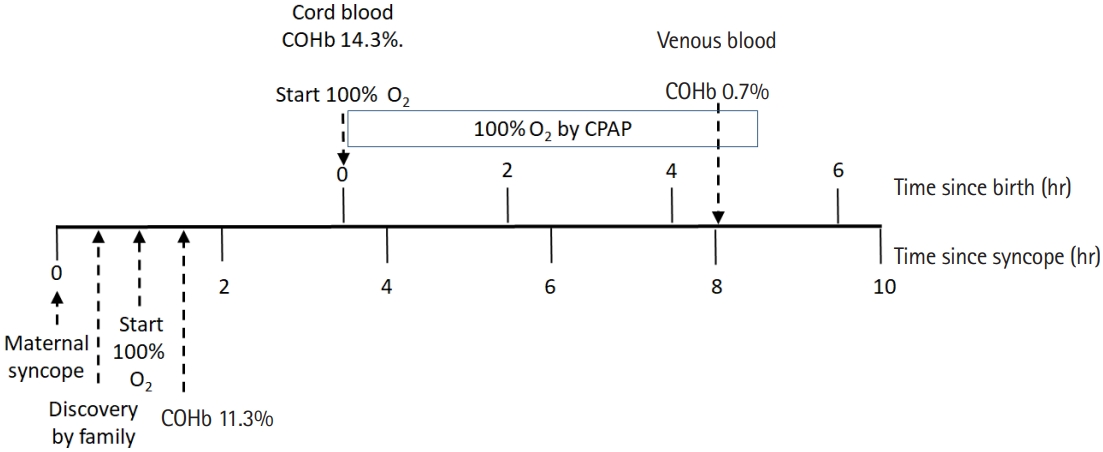
The mother reported a headache that rapidly worsened over an hour soon after a technician had been working on pipes and insulation in the attic of the residence earlier that day. She called a family member for assistance. Her relative found her confused, nauseated, and lying on the kitchen floor about half an hour later and summoned an ambulance. Paramedics recorded syncope, headache, lightheadedness, and nausea. She denied chest pain, dyspnea, contractions, or vaginal bleeding. Blood glucose was 97 mg/dL (5.4 mmol/L). In the emergency department, she additionally reported paresthesia of lips, hands, and feet. She was sleepy but oriented, tachycardic (122 beats/min), and normotensive with room air oxygen saturation of 96%. Arterial blood gases after 30 minutes of 100% oxygen (15 L/min by nonrebreather mask) and a total of 90 minutes after cessation of CO exposure included COHb of 11.3%, pH of 7.44, pCO2 of 29 mmHg, and pO2 of 287 mmHg. Her white blood cell count was 10.4 K/mcL, hemoglobin 9.1 g/dL, and bicarbonate 20 mmol/L. Urine drug screen was positive only for cannabinoids. Electrocardiography indicated normal sinus rhythm with sinus arrhythmia. Her cervix was 4 cm dilated and 70% effaced, with uterine contractions at 5-minute intervals. Fetal heart tracing was category III, with a rate of 130 beats/min, minimal variability, and occasional variable decelerations. Fetal tocometry improved to category II after 60 minutes of 100% fraction of inspired oxygen (FiO2), but concern for fetal distress compelled urgent caesarean section. Delivery of the baby boy was uncomplicated, with spontaneous cry, 60 seconds of delayed cord clamping, Apgar scores of 8 and 9, and a birth weight of 3,460 g. Time of birth was 3.5 hours after cessation of CO exposure. He immediately received continuous positive airway pressure at 6 cmH20 and 100% FiO2. Venous cord blood gases included COHb of 14.3%, pH of 7.36, pCO2 of 42 mmHg, pO2 of 21 mmHg, and base excess of –2. He was transferred to a pediatric hospital with a neonatal intensive care unit (NICU), where capillary blood gases at 4.5 hours of life showed pH of 7.43, pCO2 of 31 mmHg, pO2 of 103 mmHg, and base excess of –2.9. COHb had fallen to 0.7% and methemoglobin was 0.5%. He transitioned to room air. He fed appropriately, and we removed his umbilical venous catheter the next day. He went home on the 3rd day of life. The clinical course is summarized in Fig. 1.
Retrospectively, we estimate a peak COHb of 19% in the mother, but her history and symptoms (tachycardia, confusion, headache, and alteration in consciousness) suggest a COHb concentration in the range of 30% to 50% [4]. Importantly, blood COHb concentrations commonly do not correlate with tissue concentrations, initial symptoms, or patient outcomes [7]. Poor fetal outcomes may occur even despite maternal survival [8–10]. Symptoms of CO poisoning in infants and young children may be more difficult to detect. A case series of CO poisoning in children aged 4 days to 19 months surprisingly found a higher mean COHb in five asymptomatic patients (24%) than in seven symptomatic patients (16%) [11].
Hyperbaric oxygen therapy (HBOT) is safe for pregnant women with CO poisoning [12]. Guidelines recommend HBOT for all CO poisonings in pregnant women regardless of clinical status or COHb concentration [13]. Recommendations for neonatal HBOT are based on expert consensus. Liebelt [14] suggested infants younger than 6 months who present with lethargy, irritability, or poor feeding and have a known CO exposure for which an adult receives HBOT, should also receive HBOT. The Israeli Naval Medical Institute employs HBOT for CO-poisoned children of any age with either evidence of myocardial ischemia, COHb greater than 25%, or any neurologic symptom [15].
Risks of HBOT in neonates include seizures from central nervous system oxygen toxicity, hypothermia, potential premature closure of the ductus arteriosus, transport-related decompensation, and barotrauma. A chest radiograph should be obtained prior to HBOT to rule out neonatal lobar emphysema or pneumothorax. Myringotomy, sometimes considered for patients too young to reliably equalize middle ear pressure via the Eustachian tubes or with active middle ear effusions, is usually not necessary if the child is able to breastfeed or suck a pacifier during chamber pressurization [11]. To prevent distress or regurgitation, infants should be burped prior to ascent in the chamber.
In our case, fetal distress compelled urgent delivery. The interfacility transfer decision involved a choice between NICU services at a nearer tertiary care pediatric hospital and a more distant community hospital with HBOT. Difficulty arranging HBOT in our region, additional transport time, and the location of NICU services favored transport to the former. At 4.5 hours of life the infant had a normal COHb level and a normal neurologic exam. However, HBOT remains the recommended treatment for all pregnant women poisoned with CO, and future studies should address assessment of CO-poisoned neonates for delayed neurologic sequelae. This case supports the belief that fetal COHb is higher than maternal COHb.