AbstractExtracorporeal membrane oxygenation (ECMO) has been increasingly employed in the emergency department for patients with a potentially reversible cause of cardiac arrest. We present the case of a young female patient with an in-hospital cardiac arrest who was found to have severe right heart strain on point-of-care ultrasound (POCUS), suggesting a massive pulmonary embolism. Rapid bedside diagnosis using ultrasound expedited bedside cannulation and initiation of ECMO as a bridge to surgical thrombectomy, and ultimately the patient survived with full neurologic function. With its ready availability and increasing acceptance by consultants, POCUS should be incorporated into cardiac arrest algorithms as the standard of care to rule in thrombotic and obstructive causes of cardiac arrest.
INTRODUCTIONExtracorporeal membrane oxygenation (ECMO) has been increasingly employed in the emergency department (ED) for patients with a potentially reversible cause of cardiac arrest, such as myocardial infarction and pulmonary embolism (PE). If successful, ECMO cannulation allows time for diagnostic imaging and life-saving interventions such as cardiac catheterization or emergent thrombectomy. Chen et al. [1] reported a 34.1% rate of survival to hospital discharge in patients who underwent in-hospital cardiac arrest (IHCA) and underwent extracorporeal cardiopulmonary resuscitation (ECPR) after 10 minutes of conventional cardiopulmonary resuscitation without return of spontaneous circulation (ROSC). The CHEER (Mechanical CPR, Hypothermia, ECMO and Early Reperfusion) trial by Stub et al. [2] showed a neurologic survival rate of 54% in 26 patients who were placed on ECMO during cardiac arrest. Additionally, the mean duration of cardiopulmonary resuscitation prior to initiation of ECMO is strongly correlated with survival. Table 1 [1–9] summarizes the survival to hospital discharge rates between ECMO and traditional CPR. One retrospective study of 133 ECMO patients found low flow time to be an independent predictor of mortality, with mean CPR duration of 49.6±5.6 minutes for patients experiencing IHCA, compared to 72.2±7.4 minutes for patients with out-of-hospital cardiac arrest [10].
Although not traditionally included in the Advanced Cardiac Life Support (ACLS) algorithms, point-of-care ultrasound (POCUS) has increasingly become the standard of care in the management of cardiac arrest. The challenge for emergency physicians in these cases is the rapid identification of an underlying cause, which can be difficult in patients with minimal history and hemodynamic instability which does not allow for cross-sectional imaging. With increased implementation of diagnostic bedside ultrasound by emergency physicians, consultants have begun to trust this modality of imaging and its bedside interpretation to direct care towards faster interventions for the patient. In this case, we demonstrate the important role POCUS played in identifying severe right ventricular dysfunction as a likely cause of the cardiac arrest and helped direct a decision for urgent ECMO cannulation in the ED.
CASE REPORTThe patient was a 39-year-old female with morbid obesity who presented to the ED via ambulance with nonspecific chest and abdominal pain. On initial evaluation, she was alert but with altered mentation and distress of unclear etiology. Her heart rate was 123 beats/min with a blood pressure of 123/52 mmHg. She had been placed by emergency medical services on 6 L/min of oxygen via nasal cannula with an oxygen saturation of 96%. After triage she was placed in a resuscitation room, where she was placed on cardiac monitoring and a peripheral intravenous line was established. An electrocardiogram performed at bedside was unremarkable aside from sinus tachycardia. However, at this time she was noted to be hypotensive, intravenous fluids were administered without significant improvement, and a norepinephrine infusion was initiated. Continued hypotension required escalating doses of vasopressor medications, ultimately requiring norepinephrine (30 μg/min), vasopressin (0.04 μg/min), and epinephrine (10 μg/min). A right internal jugular central line was placed. Point-of-care troponin was elevated at 0.209 ng/mL (upper limit of normal 0.04). Due to undifferentiated hypotension, a POCUS was performed which showed significant right ventricular dilation on the parasternal long axis (Fig. 1A) and septal flattening with “D” sign on parasternal short axis (Fig. 1B), concerning in this clinical picture for massive PE.
At this time, the PE response team was consulted, as well as the ECMO team given her worsening hypotension, severe right heart strain on ultrasound, and escalating vasopressor requirements. Shortly after ECMO team arrival, she experienced a cardiac arrest. During the 29-minute period of active CPR, she was given tissue plasminogen activator (tPA) 50 mg, and under ultrasound guidance, she was placed on femoral artery-femoral vein peripheral ECMO. She rapidly stabilized on ECMO and was taken for computed tomography pulmonary angiography, which revealed a near-occlusive saddle PE. The next day she was taken for thrombectomy with interventional radiology, which was not successful due to the high burden of organized thrombus. This was followed by an open thrombectomy with cardiothoracic surgery, at which time a large thrombus was able to be removed from the pulmonary arteries (Fig. 2). She ultimately survived the procedure and during an extended hospital course she was able to be transitioned from ECMO to right ventricular assist device, and subsequently was decannulated and discharged to an acute rehabilitation unit with no neurological sequelae.
DISCUSSIONThis case illustrates the importance of POCUS as a definitive diagnostic modality for emergent decision-making in the ED. In cases of critically ill patients who are not able to undergo cross-sectional imaging, POCUS may be the only means by which the decision is made to initiate life-saving measures such as ECMO or tPA in reversible causes of cardiac arrest. Making this diagnosis at bedside can bypass a time-consuming computed tomography scan and reduce rates of decompensation in the radiology department, while providing enough information to allow the ED physician to assemble a multidisciplinary team at the bedside.
The guidelines for management of PE from the American College of Emergency Physicians (ACEP) show that “the finding of right ventricular dysfunction on bedside echocardiography may be used as indirect evidence for presence of PE, although this technology or skill level is unavailable in most EDs” [11]. With the advent of emergency provider training in POCUS, ED providers can now easily screen for the presence of right heart strain at the bedside [12]. Both the 2020 American Heart Association (AHA) guidelines and the Korean CPR guidelines state that a physician experienced in ultrasound may use this as an adjunct as long as it does not interfere with standard CPR, with the caveat that the physician should use caution to avoid interruptions in compressions [13,14].
Several features may be identified on bedside echocardiograms to screen for patients at higher risk of mortality from PE. In this case, a parasternal short view revealed a grossly dilated right ventricle as well as interventricular flattening, also termed a “D” sign (Fig. 1B). In a retrospective analysis of 511 patients, Kurnicka et al. [15] found that all hemodynamically unstable patients with PE had some degree of right ventricular dilation. Another important finding on bedside ultrasound is right heart thrombus. Both Torbicki et al. [16] and Rose et al. [17] evaluated the mortality and outcomes of patients found to have right heart thrombus on echo, finding a 21% mortality rate of those with a thrombus compared to 11% mortality of those without. Additionally, the mortality rate of those able to undergo thrombectomy with right heart thrombus compared to those who received thrombolytics alone decreased from 23.8% to 11.3%, with thrombolytics showing mortality benefit on multivariate analysis. These findings, in addition to McConnell’s sign and reduced tricuspid annular plane systolic excursion can provide evidence of right heart strain to ED physicians and consultants to allow for faster recognition of massive PE and improve mortality rates. These sonographic findings of right heart strain are summarized in Fig. 3.
Currently, POCUS is not included in the traditional AHA guidelines for ACLS algorithm. International PE management guidelines do recommend use of ultrasound in hemodynamically unstable patients with suspected PE [18]. With its ready availability and increasing acceptance by consultants, bedside cardiac echocardiography should be incorporated in cardiac arrest algorithms in order to fully evaluate the patient for thrombotic or obstructive causes of cardiac arrest, including massive PE and pericardial effusion. If these are identified quickly, there is demonstrated benefit in initiating ECMO in the ED.
ECMO has been demonstrated to be successful in select patients who experience cardiopulmonary arrest secondary to massive PE as a bridge to definitive intervention [19]. Chen et al. [3] performed an observational study and propensity analysis comparing survival rates in patients with IHCA, finding a survival rate of 23.7% in those receiving ECPR compared to 10.7% of those receiving traditional CPR. This may reflect some selection bias as those chosen for ECPR are typically younger with potentially reversible causes of cardiac arrest [20]. Another meta-analysis showed a 13% absolute risk difference in survival to hospital discharge, favoring ECPR [4]. In nine selected studies involving both IHCA and OHCA, initiation of ECMO demonstrates almost double the survival rate compared to conventional CPR (Table 1).
A series of recent studies have investigated the use of ECMO in the ED with diverse results. The ARREST (Advanced Reperfusion Strategies for Refractory Cardiac Arrest) trial enrolled 30 patients with OHCA and refractory ventricular fibrillation. They found that 1 of 15 patients (7%) in the ACLS-only group survived to hospital discharge compared to 6 of 14 (43%) in the ECMO-facilitated resuscitation group. The trial was terminated early due to the significant superiority in the ECMO group [7]. A larger trial, the Prague OHCA trial, found a 180-day survival rate of 1.2% in patients without prehospital ROSC treated with conventional ACLS and 23.9% in patients without prehospital ROSC treated with ECPR, concluding a benefit to those with OHCA without prehospital ROSC [8]. Most recently, Suverein et al. [9] performed a trial of patients with OHCA, finding that 14 of 70 patients (20%) in the ECPR group survived with favorable neurologic outcome, versus 10 of 62 in the conventional CPR group, finding no statistical difference in neurologic outcome.
Although ED ECMO is still under ongoing clinical investigation, there is literature to support the benefit of ED ECMO in select patients. In these cases, every effort must be made to identify the cause of the cardiac arrest and in suitable candidates, rapid placement on ECMO is advisable. The ability to perform POCUS to identify these causes efficiently can add an important role in the decision-making and survival of these patients.
NOTESREFERENCES1. Chen YS, Yu HY, Huang SC, et al. Extracorporeal membrane oxygenation support can extend the duration of cardiopulmonary resuscitation. Crit Care Med 2008; 36:2529-35.
2. Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation 2015; 86:88-94.
3. Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008; 372:554-61.
4. Shin TG, Jo IJ, Sim MS, et al. Two-year survival and neurological outcome of in-hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int J Cardiol 2013; 168:3424-30.
5. Ouweneel DM, Schotborgh JV, Limpens J, et al. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med 2016; 42:1922-34.
6. Dennis M, Buscher H, Gattas D, et al. Prospective observational study of mechanical cardiopulmonary resuscitation, extracorporeal membrane oxygenation and early reperfusion for refractory cardiac arrest in Sydney: the 2CHEER study. Crit Care Resusc 2020; 22:26-34.
7. Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 2020; 396:1807-16.
8. Rob D, Smalcova J, Smid O, et al. Extracorporeal versus conventional cardiopulmonary resuscitation for refractory out-of-hospital cardiac arrest: a secondary analysis of the Prague OHCA trial. Crit Care 2022; 26:330.
9. Suverein MM, Delnoij TS, Lorusso R, et al. Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N Engl J Med 2023; 388:299-309.
10. Wengenmayer T, Rombach S, Ramshorn F, et al. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR). Crit Care 2017; 21:157.
11. Fesmire FM, Brown MD, Espinosa JA, et al. Critical issues in the evaluation and management of adult patients presenting to the emergency department with suspected pulmonary embolism. Ann Emerg Med 2011; 57:628-52.
12. Daley JI, Dwyer KH, Grunwald Z, et al. Increased sensitivity of focused cardiac ultrasound for pulmonary embolism in emergency department patients with abnormal vital signs. Acad Emerg Med 2019; 26:1211-20.
13. Panchal AR, Bartos JA, Cabanas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020; 142:S366-468.
14. Hwang SO, Cha KC, Jung WJ, et al. 2020 Korean guidelines for cardiopulmonary resuscitation. Part 1: update process and highlights. Clin Exp Emerg Med 2021; 8:S1-7.
15. Kurnicka K, Lichodziejewska B, Goliszek S, et al. Echocardiographic pattern of acute pulmonary embolism: analysis of 511 consecutive patients. J Am Soc Echocardiogr 2016; 29:907-13.
16. Torbicki A, Galie N, Covezzoli A, et al. Right heart thrombi in pulmonary embolism: results from the International Cooperative Pulmonary Embolism Registry. J Am Coll Cardiol 2003; 41:2245-51.
18. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3033-3069k.
Fig. 1.Echocardiographic views. (A) Parasternal long axis view with right ventricular dilation (asterisk). (B) Parasternal short axis view demonstrating right ventricular dilation and septal flattening (arrow). 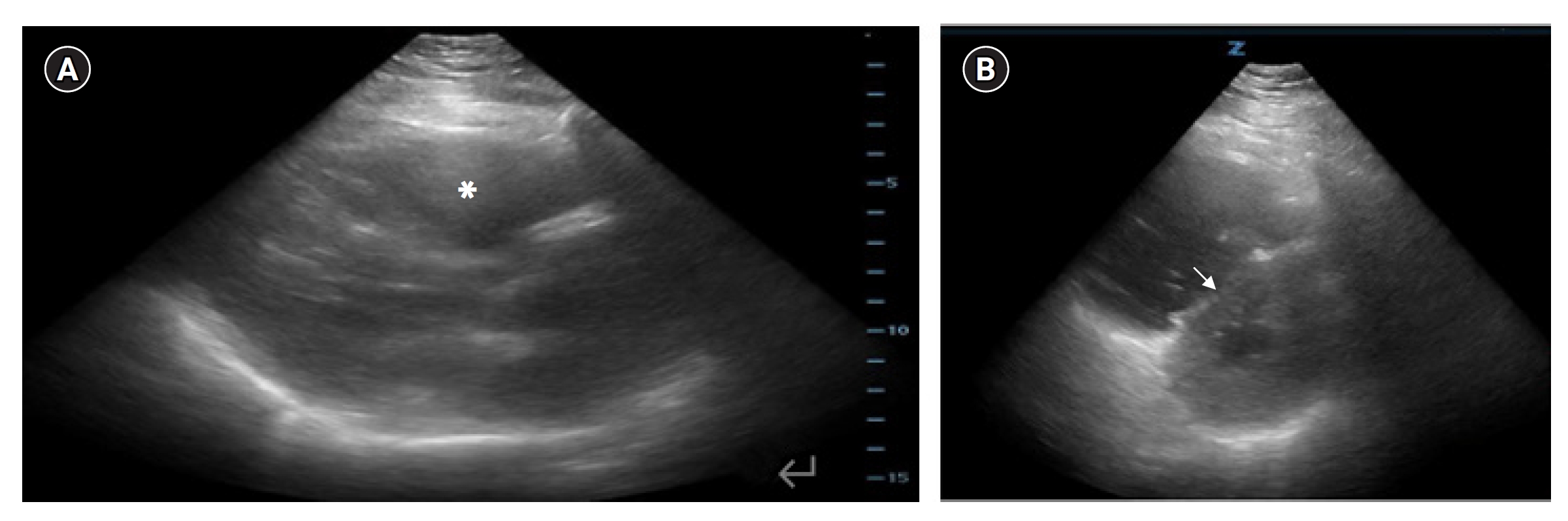
Table 1.Survival to hospital discharge rates of ECMO and traditional CPR
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||