AbstractObjectiveThe predictors of poor prognosis in heat stroke (HS) remain unknown. This study investigated the predictive factors of poor prognosis in patients with HS.
MethodsData were obtained and analyzed from the health records of patients diagnosed with heat illness at Ajou university hospital between January 2008 and December 2017. Univariate and multivariate analyses were performed to identify the independent predictors of poor prognosis.
ResultsThirty-six patients (median age, 54.5 years; 33 men) were included in the study. Poor prognosis was identified in 27.8% of the study population (10 patients). The levels of S100B protein, troponin I, creatinine, alanine aminotransferase, and serum lactate were statistically significant in the univariate analysis. Multiple regression analysis revealed that poor prognosis was significantly associated with an increased S100B protein level (odds ratio, 177.37; 95% confidence interval, 2.59 to 12,143.80; P=0.016). The S100B protein cut-off level for predicting poor prognosis was 0.610 μg/L (area under the curve, 0.906; 95% confidence interval, 0.00 to 1.00), with 86% sensitivity and 86% specificity.
INTRODUCTIONRecently, the occurrence rate of heat-related diseases has increased as the average annual temperature has risen globally [1,2]. The increase in the occurrence of heat-related illness secondary to global warming has become a threat to public health, especially among the elderly population [1,3-7]. Among heat-related illnesses (HRIs), heat stroke (HS) is classic and the most serious form of heat injury [8].
According to Kalaiselvan et al. [5] the incidence of HS, a leading cause of hospital mortality related to global warming, is also increasing [1,6]. HS is defined as a core temperature greater than 40°C and the occurrence of central nervous system abnormalities [7-9]. HS is largely divided into classic HS secondary to high external temperature and exertional HS secondary to physical exertion. The pathophysiology of HS involves the activation of numerous inflammatory and hemostatic pathways causing a systemic inflammatory response syndrome and multi-organ dysfunction syndrome [7,8,10,11]. Since progression of the disease is relatively rapid, rapid diagnosis and treatment are vital [12-17]. Severe damage to the central nervous system can result in permanent neurological disability or death. Moreover, the reported mortality is as high as 71% [7,8].
According to Zhao et al., disseminated intravascular coagulation and acute kidney injury are the predictors of major mortality in relation to the prognosis of HS [17-20]. Decreased mental status and delayed transport time from the scene to the final treatment hospital are also poor prognostic factors [1,12,13,15,21]. In association with procalcitonin, interleukin-6 and tumor necrosis factor-alpha are associated with poor prognosis, whereas melatonin can reduce the degree of HS [11,19,22-25]. However, these results are from relatively small studies, case reports, or laboratory experimental studies; therefore, further research is needed.
The S100B protein has a molecular weight of 21 kDa and a half-life of 30 minutes [26,27]. It plays a significant role in predicting the prognosis of various disease groups including traumatic brain injury [28-34]. Considering the pathophysiology of HS, the S100B protein might also be useful in predicting the prognosis of HS.
Nevertheless, the predictors of poor prognosis for HS remain unknown. The purpose of this study was to evaluate the predictors of poor prognosis in patients with HS.
METHODSStudy design and settingThis study was conducted retrospectively by collecting data from patients who visited an emergency medical center of a university-affiliated hospital between January 2008 and December 2017. The study was approved by Ajou Institutional Review Board (AJIRB-MED-MDB-18-282) and was exempt from the informed consent requirement.
The inclusion criterion was a confirmed diagnosis of consecutive HS. HS was defined when the patient had a history of exposure to a high-temperature environment and one or more of the following central nervous system manifestations: Glasgow Coma Scale (GCS) score <8, cerebellar symptoms, convulsions, and seizures. The diagnosis was established according to the patient’s history, clinical characteristics, physical examination, or body core temperature >40°C. The exclusion criteria were as follows: age <18 years, a previous diagnosis of a neuropsychiatric disease, a postcardiac arrest state, current head trauma, or melanoma, which could lead to an elevated serum S100B level. The patients included in this study were divided into those with a good prognosis or those with a poor prognosis. A poor prognosis was defined as the condition in which the patient could not live without assistance at hospital discharge (cerebral performance category score ≥3).
Data collectionStandardized extraction of demographic, clinical, laboratory, and radiological data from medical records was performed by two trained emergency physicians. The collected data included age; sex; pre-existing diseases; GCS score at admission; vital signs including maximum body core temperature from the scene to hospital admission, serum blood urea nitrogen level, creatinine level (mg/dL), aspartate aminotransferase/alanine aminotransferase level, creatine phosphokinase level, troponin I level, and partial thromboplastin/activated partial thromboplastin time; and poor prognosis including hospital mortality.
Any discrepancy between the datasets extracted by the two emergency physicians was resolved by a third physician. Laboratory studies including evaluation of the serum S100B protein level were performed when the patient visited the emergency department. The serum S100B protein level was measured using a quantitative immunoassay analyzer (Modular Analytics E170; Roche Diagnostics, Indianapolis, IN, USA). The cut-off value provided by the manufacturer was 0.105 μg/L, and the lower detection limit was 0.005 μg/L.
Statistical analysisAll data are expressed as means±standard deviation or as medians (interquartile range), as appropriate. The significance of intergroup differences was assessed by using Fisher exact tests for categorical variables and Mann-Whitney U-tests for continuous variables. Multiple logistical regression analysis was performed to identify the factors that could be considered independent predictors of poor prognosis after HRI using the forward stepwise method with the likelihood ratio test. We constructed a multivariate model using variables that were selected from the univariate analysis (P<0.05) and factors known to be associated with factors of poor prognosis after HRI: the time from the highest body temperature to normal body temperature and levels of serum S100B protein, creatinine, alanine aminotransferase, lactate, and troponin I. A P-value <0.05 was considered statistically significant. Receiver operating characteristic curves were constructed to establish the cut-off points for the serum S100B protein level with the optimal sensitivity and specificity in predicting poor prognostic factors in patients with HS. The statistical analysis was performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTSForty-eight patients with HS were included in the present study. After excluding 12 patients with missing data of the S100B protein level, 36 patients were finally included.
Baseline patient characteristicsPatients’ median age was 54.5 (41.8–64) years, and 33 patients (91.7%) were men. The incidence of poor prognosis was 27.8% (10 patients including 2 patients who died). Eighteen patients each had classic and exertional-type HS. Other patient characteristics are shown in Table 1.
Comparisons of characteristics between the good and poor prognosis groupsThe S100B protein levels were significantly higher in the poor prognosis group than in the good prognosis group (median [interquartile range], 0.95 [0.62 to 2.52] vs. 0.20 [0.14 to 0.47]; P<0.001) (Fig. 1). There were statistically significant differences in the serum levels of creatine, lactate, troponin I, aspartate aminotransferase, and alanine aminotransferase and the systolic blood pressure between the two groups (Table 2).
Analysis of factors associated with poor prognosisMultivariate logistic regression analysis showed that only the serum S100B protein was independently associated with poor prognosis in patients with HS (B 5.18; S.E. 2.16; 95% CI 2.59-12,143.80; P=0.016). The optimal cut-off value of the S100B protein level for predicting poor prognosis was 0.610 μg/L (Fig. 2). The sensitivity and specificity for this cut-off value were 86% and 86%, respectively. The receiver operating characteristic curve is shown in Fig. 2.
DISCUSSIONIn the present study, we observed that the S100B protein level was increased in patients with HS. In addition, an S100B protein level >0.61 μg/L predicted poor prognosis in these patients. An increase in the S100B protein level is a poor prognostic factor in diseases associated with the brain, including stroke, traumatic brain injury, and acute carbon monoxide poisoning [28-34]. The S100B protein is also a factor in predicting the prognosis and degree of injury in various diseases in which brain injury occurs, and it showed potential usefulness as a prognostic factor in this study. The S100B protein concentration in the poor prognosis group was 0.95 (0.62 to 2.52) μg/L, which is about five times higher than the value (0.20 [0.14 to 0.47]) μg/L in the good prognosis group. Considering the fact that the pathophysiology of HS is multiorgan injury accompanied by a systemic inflammatory response syndrome and acute brain injury occurs because of a high core temperature, our result is important and meaningful, as it suggests that the S100B protein is a prognostic factor with regard to HS.
Our results showed that the S100B protein level, age, GCS score, systolic blood pressure, prothrombin time, activated partial thromboplastin time, troponin I level, and creatinine level, which are known prognostic factors for HS, were not independent prognostic factors for HS in the logistic regression analysis, although they were statistically significant in the univariate analysis [15,17-19,35-37]. These results were contrary to those of previous studies. Further research is needed to confirm these findings because our study had a small sample size and did not differentiate between exertional and classic-type HS.
Regarding the baseline patient characteristics, the sauna/bathroom was the most common situational risk factor in classic HS. In the sauna and bathroom, as humidity increases, the effectiveness of evaporative cooling from sweating decreases. In these instances, sweating only exacerbates dehydration, which might encourage the development of classic HS [38]. Classic HS occurs irrespective of hot weather or the season if the patient is exposed to hot and humid conditions such as those in a sauna. Therefore, public saunas and bathrooms are important risk factors for the development of classic HS.
Identification of prognostic factors in the early stage of HS is important to achieve the optimal therapeutic effect because it is the basis on which prompt treatment can be started [7,12,16,21,39]. Early identification of S100B protein levels in the emergency department as a prognostic factor might play a role in the active treatment of patients with HS in its early stage. High S100B protein levels are likely to indicate a critical condition requiring prompt treatment in patients with HS because a rapid decrease in core body temperature is critical to preventing the progression of brain injury in patients with HS. Therefore, measurement of the S100B protein levels will be helpful in further studies on brain injuries associated with HRI.
The limitations of our study are as follows. First, although the study period was relatively long, a limited number of subjects was included in the present study; therefore, our findings cannot be generalized. Additionally, we excluded 12 patients because of missing S100B protein data. However, we believe that our report is the first to show the association of the S100B protein level with HS as a prognostic factor. Second, follow-up assessment of the S100B protein level was conducted in a small number of patients in this study; thus, we could not investigate how great a decrease in body core temperature affected a decrease of the S100B protein level. Future prospective studies are required to investigate this topic. Third, although our study was conducted at the final treatment hospital, it was difficult to confirm the final recovery of some patients because of transfers to other hospitals for rehabilitation treatment, which may have affected the outcomes. Lastly, the chart analysis was retrospective.
In conclusion, to our knowledge, this is the first study to evaluate the S100B protein level as a prognostic factor in HS. An increased S100B protein concentration was a prognostic factor for HS in the present study. Although the pathophysiology of HS remains to be elucidated, the possibility of S100B protein as a prognostic factor in HS is worth investigating to determine the best treatment of HS. Early identification of poor prognostic factors and rapid treatment may improve the prognosis in patients with HS. However, further research is required to confirm our findings.
REFERENCES1. Yang J, Zhou M, Li M, et al. Vulnerability to the impact of temperature variability on mortality in 31 major Chinese cities. Environ Pollut 2018; 239:631-7.
2. Zhang K, Chen TH, Begley CE. Impact of the 2011 heat wave on mortality and emergency department visits in Houston, Texas. Environ Health 2015; 14:11.
3. Morch SS, Andersen JD, Bestle MH. Heat stroke: a medical emergency appearing in new regions. Case Rep Crit Care 2017; 2017:6219236.
4. Zhao YJ, Hu XD, Huang YB, Wang WP, Yang MJ. A study on the incidence of heat stroke and explore it’s prediction model in Pudong New Area of Shanghai from 2013-2017. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2018; 36:285-7.
5. Kalaiselvan MS, Renuka MK, Arunkumar AS. A retrospective study of clinical profile and outcomes of critically ill patients with heat-related illness. Indian J Anaesth 2015; 59:715-20.
6. Berlot G, Marcer G, Zornada F, Nieto Yabar M, Tomasini A, Iscra F. Heat stroke: clinical experience from an Italian ICU during summer 2015. Eur J Intern Med 2016; 33:e11-2.
9. People’s Liberation Army Professional Committee of Critical Care Medicine. Expert consensus on standardized diagnosis and treatment for heat stroke. Mil Med Res 2016; 3:1.
10. Adams T, Stacey E, Stacey S, Martin D. Exertional heat stroke. Br J Hosp Med (Lond) 2012; 73:72-8.
11. Tong HS, Liu YS, Wen Q, Tang YQ, Yuan FF, Su L. Serum procalcitonin predicting mortality in exertional heatstroke. Emerg Med J 2012; 29:113-7.
12. Stearns RL, Casa DJ, O’Connor FG, Lopez RM. A tale of two heat strokes: a comparative case study. Curr Sports Med Rep 2016; 15:94-7.
13. Pan ZG, Shao Y, Liu YN, et al. Relationship between early coagulability parameters at admission and outcome in patients with severe heatstroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013; 25:725-8.
15. Hemmelgarn C, Gannon K. Heatstroke: clinical signs, diagnosis, treatment, and prognosis. Compend Contin Educ Vet 2013; 35:E3.
16. Wasserman DD, Healy M. Cooling techniques for hyperthermia. Treasure Island, FL: StatPearls Publishing; 2018.
17. Zhao JJ, Zhou JJ, Hu J, et al. Analysis of risk factors affecting prognosis of exertional heat stroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013; 25:515-8.
18. Fan H, Zhao Y, Zhu JH, et al. Thrombocytopenia as a predictor of severe acute kidney injury in patients with heat stroke. Ren Fail 2015; 37:877-81.
19. Varghese GM, John G, Thomas K, Abraham OC, Mathai D. Predictors of multi-organ dysfunction in heatstroke. Emerg Med J 2005; 22:185-7.
20. Hifumi T, Kondo Y, Shimazaki J, et al. Prognostic significance of disseminated intravascular coagulation in patients with heat stroke in a nationwide registry. J Crit Care 2018; 44:306-11.
21. Lou Y, Wang H, Li H, Chen W, Sha N. Impact of exertional heat stroke treatment time on prognosis: a report of 2 cases. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016; 28:744-6.
22. Heled Y, Fleischmann C, Epstein Y. Cytokines and their role in hyperthermia and heat stroke. J Basic Clin Physiol Pharmacol 2013; 24:85-96.
23. Hausfater P, Hurtado M, Pease S, et al. Is procalcitonin a marker of critical illness in heatstroke? Intensive Care Med 2008; 34:1377-83.
24. Wu WS, Chou MT, Chao CM, Chang CK, Lin MT, Chang CP. Melatonin reduces acute lung inflammation, edema, and hemorrhage in heatstroke rats. Acta Pharmacol Sin 2012; 33:775-82.
25. Alzeer AH, el-Hazmi MA, Warsy AS, Ansari ZA, Yrkendi MS. Serum enzymes in heat stroke: prognostic implication. Clin Chem 1997; 43:1182-7.
26. Moore BW. Chemistry and biology of two brain-specific proteins, s-100 and 14-3-2. Adv Exp Med Biol 1972; 32:5-7.
28. Woertgen C, Rothoerl RD, Metz C, Brawanski A. Comparison of clinical, radiologic, and serum marker as prognostic factors after severe head injury. J Trauma 1999; 47:1126-30.
29. Alatas OD, Gurger M, Atescelik M, et al. Neuron-specific enolase, S100 calcium-binding protein B, and heat shock protein 70 levels in patients with intracranial hemorrhage. Medicine (Baltimore) 2015; 94:e2007.
30. Weiss N, Sanchez-Pena P, Roche S, et al. Prognosis value of plasma S100B protein levels after subarachnoid aneurysmal hemorrhage. Anesthesiology 2006; 104:658-66.
31. Rezaei O, Pakdaman H, Gharehgozli K, et al. S100 B: a new concept in neurocritical care. Iran J Neurol 2017; 16:83-9.
32. Ballesteros MA, Rubio-Lopez MI, San Martin M, et al. Serum levels of S100B from jugular bulb as a biomarker of poor prognosis in patients with severe acute brain injury. J Neurol Sci 2018; 385:109-14.
33. Yao B, Zhang LN, Ai YH, Liu ZY, Huang L. Serum S100β is a better biomarker than neuron-specific enolase for sepsis-associated encephalopathy and determining its prognosis: a prospective and observational study. Neurochem Res 2014; 39:1263-9.
34. Park E, Ahn J, Min YG, et al. The usefulness of the serum S100B protein for predicting delayed neurological sequelae in acute carbon monoxide poisoning. Clin Toxicol (Phila) 2012; 50:183-8.
35. Miao L, Song Q, Liu H, et al. Correlation between gastrointestinal dysfunction and both severity and prognosis in patients suffering from heatstroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015; 27:635-8.
36. Carvalho AS, Rodeia SC, Silvestre J, Povoa P. Exertional heat stroke and acute liver failure: a late dysfunction. BMJ Case Rep 2016; 2016.
37. Hausfater P, Megarbane B, Fabricatore L, et al. Serum sodium abnormalities during nonexertional heatstroke: incidence and prognostic values. Am J Emerg Med 2012; 30:741-8.
Fig. 1.Comparison of S100B protein levels between the good and poor prognosis groups. The box plots show the median S100B protein levels in each group. The median level in the poor prognosis group is about five times higher than that in the good prognosis group (0.95 [0.62 to 2.52] vs 0.20 [0.14 to 0.47] µg/L; P<0.001). 
Fig. 2.Receiver operating characteristics curve for the S100B protein level and poor prognosis of heat stroke. An S100B protein level cut-off of 0.610 µg/L predicted poor prognosis in patients with heat stroke with 86% sensitivity and 86% specificity (area under the curve, 0.91 [95% confidence interval, 0.79 to 1.00]; p=0.001). 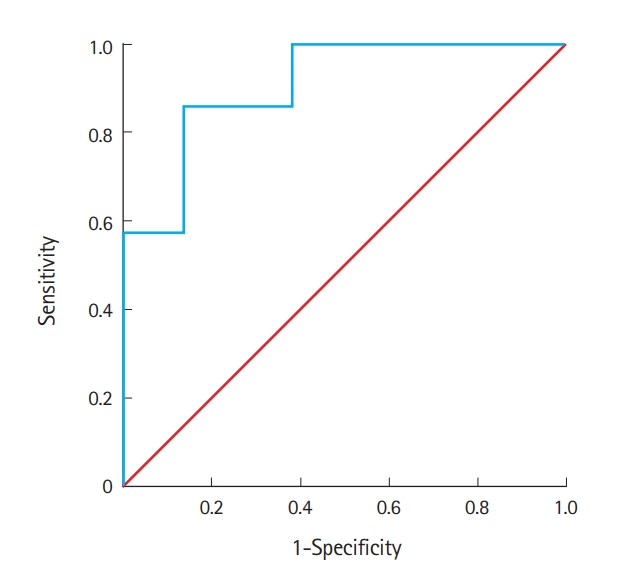
Table 1.Baseline patient characteristics Table 2.Comparisons of clinical characteristics between the good and poor prognosis groups Values are presented as median (interquartile range). GCS, Glasgow Coma Scale; ED, emergency department; SBP, systolic blood pressure; BT, body temperature; Hb, hemoglobin; BUN, blood urea nitrogen; Cr, creatinine; CK, creatine phosphokinase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PT, prothrombin time; aPTT, activated partial thromboplastin time; CRP, C reactive protein. |
|
||||||||||||||||||||||||||||||||||||||||